Abstract
Introduction:
Since 2004, several jurisdictions have mandated that cigarettes show reduced ignition propensity (RIP) in laboratory testing. RIP cigarettes may limit fires caused by smoldering cigarettes, reducing fire-related deaths and injury. However, some evidence suggests that RIP cigarettes emit more carbon monoxide and polycyclic aromatic hydrocarbons, and smokers may alter their smoking patterns in response to RIP cigarettes. Both of these could increase smokers’ exposures to harmful constituents in cigarettes.
Methods:
An 18-day switching study with a comparison group was conducted in Boston, MA (N = 77), and Buffalo, NY (N = 83), in 2006–2007. Current daily smokers completed 4 laboratory visits and two 48-hr field data collections. After a 4-day baseline, Boston participants switched to RIP cigarettes for 14 days, whereas Buffalo participants smoked RIP cigarettes throughout. Outcome measures included cigarettes smoked per day; smoking topography; salivary cotinine; breath CO; and hydroxylated metabolites of pyrene, naphthalene, phenanthrene, and fluorene. Because the groups differed demographically, analyses adjusted for race, age, and sex.
Results:
We observed no significant changes in smoking topography or CO exposure among participants who switched to RIP cigarettes. Cigarette use decreased significantly in the switched group (37.7 cigarettes/48 hr vs. 32.6 cigarettes/48 hr, p = .031), while hydroxyphenanthrenes increased significantly (555 ng/g creatinine vs. 669 ng/g creatinine, p = .007). No other biomarkers were significantly affected.
Discussion:
Small increases in exposure to phenanthrene among smokers who switched to RIP versions were observed, while other exposures and smoking topography were not significantly affected. Toxicological implications of these findings are unclear. These findings should be weighed against the potential public health benefits of adopting RIP design standards for cigarette products.
Introduction
Cigarette-caused fires are a leading cause of unintentional fire injuries and deaths—for example, in the United States in 2006, 30,400 smoking-material structure fires resulted in 780 deaths and 1,600 injuries (Hall, 2008). In response, governments have enacted legislation mandating cigarette fire safety standards that are intended to increase the likelihood that cigarettes self-extinguish when not being actively puffed upon. In June 2004, New York (NY) State became the first locality in the world to mandate a fire safety standard for all cigarettes sold in that state (Fire Safety Standards for Cigarettes, 2003). This standard requires that no more than 25% of cigarettes burn their full length when not actively puffed upon, using the American Society for Testing and Materials (ASTM) 2187-04 method for measuring ignition propensity (ASTM, 2004). By March 2010, all 50 U.S. states and the District of Columbia had passed similar fire safe cigarette laws (Coalition for Fire-Safer Cigarettes, 2010a). In 2005, Canada became the first country to adopt a national fire safety standard for cigarettes similar to that of NY (Stanwick, 2005), and Australia (McGuirk, 2009) and the European Union (Arnott & Berteletti, 2008) have announced plans to do so as well.
Current cigarette ignition propensity standards do not stipulate the cigarette design to be used in order to be compliant (Coalition for Fire-Safer Cigarettes, 2010b). Internal industry document research has demonstrated that cigarette manufacturers have for years been able to produce reduced ignition propensity (RIP) cigarettes (Gunja, Wayne, Landman, Connolly, & McGuire, 2002). Certain design parameters have the potential to reduce cigarette ignition propensity, including reduced porosity of the cigarette wrapper, reduction of burn additives, higher tobacco rod density, and smaller cigarette circumference (Alpert, O’Connor, Spalletta, & Connolly, 2010; Karter et al., 1994). However, the range of cigarette design strategies available is rarely used in practice. Instead, RIP design modifications focus on the use of lower porosity paper placed in circumferential bands at two or three positions along the cigarette rod. The bands reduce ignition propensity by decreasing airflow to the burning ember. This banded paper strategy was first introduced commercially by Philip Morris with its Merit brand in 2000 (Alpert et al.; Gunja et al.). Since that time, “banded paper” technology has become the most common way to comply with RIP regulations (Alpert et al.).
Internal tobacco industry documents as well as patents reflect manufacturers’ concern with consumer acceptability regarding the taste of RIP cigarettes, one of the primary non-safety based goals of industry research and development in this area (Alpert et al., 2010; Warwick, 2000). However, the effect of cigarette changes made to comply with RIP regulations on consumers has not been well examined, at least outside the tobacco industry. A major lesson learned from the marketing of low-tar cigarettes in the 1960s through the 1980s was that changes in cigarette design could have an untoward effect on smokers’ behaviors, resulting in altered exposure to smoke constituents (National Cancer Institute, 2001). The advantages of reducing death and injury caused by cigarette-related fires, then, could potentially be offset by increases in exposure and disease outcomes, undermining any public health advantage.
RIP design changes, by altering burn characteristics and/or human smoking patterns, could potentially influence exposures to tobacco toxicants. It is possible that self-extinguishing RIP cigarettes may lead smokers to puff more frequently and/or more vigorously in order to keep the cigarette burning, thereby increasing exposure to smoke toxins (Bavley, 2009; Goswami, 2007; Tobacco Control Programme, 2002). The chemical composition and characteristics of cigarette tar may also be different in RIP cigarettes, potentially influencing the mix of toxins to which a smoker is exposed. Connolly et al. (2005) assessed the levels of 19 toxic smoke emissions of four matched full flavor cigarette brands (Marlboro, Newport, Camel, and Kool) purchased in NY, which had a fire safety standard for cigarettes in 2005, and in MA, which did not. The smoke constituents, measured using the Federal Trade Commission machine smoking protocol, included polycyclic aromatic hydrocarbons (PAHs: naphthalene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[a]pyrene, and indeno[1, 2, 3-cd]pyrene) and “tar,” nicotine, and carbon monoxide. The largest increases in machine-yield smoke constituents among RIP brands, compared with non-RIP brands, were seen for carbon monoxide (11.4%), naphthalene (13.9%), fluorene (6.1%), and tar (3.0%). One brand, Newport, showed significant increases in most of the PAHs, suggesting that either the RIP technology used or other design features of those cigarettes may have led to observed differences. While the clinical significance of these increases in smoke constituent yields is undetermined, the observed variation between the two sets of brands was much less than the range of variation in these toxins measured across major cigarette brands (cf. Harris, 2004).
The current study sought to examine whether short-term switching from conventional to RIP cigarettes is associated with changes in smoking behavior and/or exposure to smoke constituents that suggest increased risk to the smoker. Responses from a comparison group who use the RIP product throughout the study will provide an index against which to compare the magnitude of changes observed in the switched experimental group. It was hypothesized that switching from non-RIP to RIP cigarettes would produce a change in smoking behavior, including more cigarettes smoked per day and/or increased intensity of puffing within cigarettes which in turn would produce an increase in biomarkers of exposure to carbon monoxide, cotinine, and/or PAH compounds. We focus on these constituents as they were reported to be somewhat elevated in mainstream smoke of RIP cigarettes (Connolly et al., 2005). The optimal method for assessing the influence of cigarette product design on smoking behavior and exposure to tobacco smoke constituents is the forced switching study design. This protocol uses a standard crossover experimental approach and requires participants to switch to a different cigarette product for a certain period of time after a baseline period in which smokers use their preferred product (Breland, Kleykamp, & Eissenberg, 2006; Hatsukami et al., 2009).
Methods
Participants
Parallel samples were recruited in Buffalo, NY (November 2006 through October 2007), and Boston, MA (January 2007 through July 2007). Eligible participants were aged 18 to 55 years, smoked at least five cigarettes daily, used no other tobacco or nicotine products, and reported no intention of quitting smoking within the next 30 days. To provide broad coverage of the U.S. cigarette market while limiting tested brands to a manageable number, we limited participation to users of the leading brands of the major U.S. manufacturers (Newport, Marlboro, and Camel) and required participants to use those brands exclusively throughout the study period. People reporting heart or lung disease and females who reported they might be pregnant or planned to become pregnant during the study were excluded from participation.
Design and Procedures
The Buffalo, NY, site served as a comparison group (COM), as all subjects were verified to be smoking RIP-compliant cigarettes at the commencement of the study. Participants recruited from the Boston, MA, site comprised the experimental group (EXP) and verified to be using non-RIP cigarettes prior to the study. EXP group participants switched from non-RIP to RIP-compliant versions of their usual brands during the second half of the study.
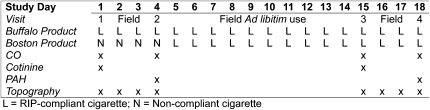
The repeated measures study design is illustrated in Figure 1. Participants were asked to visit the laboratory on four separate occasions (Days 1, 4, 15, and 18) over an 18-day period on consistent days and times of the day in order to reduce variability in daily exposures. All visits were scheduled between 7 and 11 a.m. Subjects provided informed consent on Day 1 and were requested to complete a brief baseline questionnaire regarding demographics (age, race, and gender), smoking behavior, beliefs about smoking, prior quit attempts, and risk behaviors and perceptions associated with cigarette-related fires. Participants provided a saliva sample and a pre-smoking breath sample for alveolar CO assessment. Subjects then smoked a single cigarette of their own brand in the laboratory using a CReSSmicro (Plowshare/Borgwaldt-KC, Richmond, VA) smoking topography measurement device. At the Boston site, the cigarette was smoked indoors under exemptions to smoke-free workplace laws provided by the Commonwealth of Massachusetts and the City of Boston for the purpose of conducting research. At the Buffalo site, the cigarette was smoked outdoors in accordance with the institutional smoke-free campus policy. Subjects were then instructed to smoke at least 5 cigarettes/day using the CReSSmicro device over the following 2 days (Days 2 and 3) and were requested to collect all of their cigarette butts during this time. Participants returned 2 days later (Day 4) to complete follow-up questionnaires. At this time, they provided a urine sample, two breath samples, and smoked a single cigarette in the laboratory. At the end of this visit, subjects were provided a week’s supply of their usual brand cigarettes in the RIP version, based on the amount reported smoking when enrolled in the study, and were asked to smoke only those cigarettes for the next 10 days (Days 5 through 14) until the next supply was provided. Participants then returned for Days 15 and 18, which mirrored the procedures for Days 1 and 4, respectively, with the exception that participants at both sites used the RIP cigarettes. Participants received $25 at the completion of each visit to compensate for their time. The study protocol was approved by Institutional Review Boards at Roswell Park Cancer Institute and Harvard School of Public Health.
Figure 1.
Switching study design: COM group (Buffalo, NY) and EXP group (Boston, MA) by day. RIP = reduced ignition propensity.
Products
Cigarettes for the period covering Days 1–4 were obtained in the respective cities for each of the two study arms. EXP participants received the non-RIP version of their usual brands, while COM participants received the RIP version of their usual brands. From the end of Day 4 through Day 18, participants at both sites were provided with the RIP version of their usual brands purchased from retailers in Buffalo, NY, dispersed to them in 1-week increments based on their self-reported consumption at baseline. Compliance with the protocol (i.e., use of only the assigned cigarettes) was assessed by self-report, as it was difficult to distinguish between RIP and non-RIP cigarettes after smoking.
Smoking Behavior Measures
Self-reported smoking data during each of the 2-day field periods (Days 2 and 3 and Days 16 and 17) were obtained using a daily diary. Participants were asked to record each cigarette smoked as well as the time at which each cigarette was smoked. The CReSSmicro (Plowshare/Borgwaldt-KC) device was used to assess puffing behavior during laboratory sessions and under naturalistic conditions while smokers were in the field. Key parameters measured included cigarette puff count, per puff volume (milliliter), puff velocity (milliliter per second), puff duration (millisecond), and interpuff interval (millisecond). The total puff volume (milliliter per cigarette) for each cigarette was calculated by summing all per puff volume values for that cigarette. For the current study, data from the laboratory-smoked cigarettes were considered.
Exposure Biomarker and Smoking Measures
Alveolar CO was measured using a Micro 4 Smokerlyzer (Bedfont, Kent, UK). Participants were instructed to hold their breath for 15 s before providing a sample of exhaled air, as per manufacturer’s instructions. Salivary cotinine was assayed using the enzyme-linked immunosorbent (EIA) method at Salimetrics LLC (University Park, PA). Urine was assayed for nine hydroxylated polycyclic aromatic hydrocarbon (OH-PAH) metabolites, present in human urine as glucuronide and/or sulfate conjugates, at the National Center for Environmental Health at the Centers for Disease Control and Prevention (Atlanta, GA) using a previously published method (Li et al., 2006). These biomarkers included 1-hydroxypyrene (1-OH-PYR; metabolite of pyrene), 1- and 2-naphthol (metabolites of naphthalene), 2-, 3-, and 9-hydroxyfluorene (metabolites of fluorene), and 1-, 3-, and 4-hydroxyphenanthrene (metabolites of phenanthrene). The methodology is based on enzymatic deconjugation of the analytes to yield free OH-PAHs, liquid–liquid extraction into pentene, evaporation to remove the solvent, reconstitution of the extracts in toluene, and derivitization to yield the trimethylsiloxane derivatives. Analytical determination of the target markers was performed by gas chromatography isotope dilution high-resolution mass spectrometry (Li et al., 2006). For statistical analysis, the naphthol, hydroxyfluorene, and hydroxyphenanthrene compounds were each summed to create summary exposure measures, and all metabolites are reported after adjustment for urinary creatinine concentration.
Data Analysis
Differences in demographic and smoking-related variables between the two groups at baseline were tested using chi-square and t tests. Change in smoking behavior and exposure was examined using linear mixed models with maximum likelihood estimation. Day was a repeated factor and group (COM and EXP) a fixed factor. All models controlled for age (continuous), gender (male/female), race (White, Black, and other), and brand family (Newport, Marlboro, and Camel). Biomarker models additionally adjusted for time since last cigarette (CO), cigarettes smoked the previous day (cotinine), and cigarettes smoked over past 48 hr (PAHs). Cotinine and PAH biomarkers were natural logarithm transformed prior to analysis; consequently, geometric mean values are reported. Statistical significance was accepted at p < .05, two tailed. Statistical analyses were performed using SPSS version 16 (SPSS Inc, Chicago, IL).
Results
Participant Characteristics
Demographic data for EXP and COM groups are shown in Table 1. The EXP group had a greater proportion of male participants compared with COM and had more White participants. In contrast, the COM group featured a small majority of Black participants. EXP participants were younger and reported a longer latency to smoke the first cigarette of the day. Newport brand cigarettes were overwhelmingly usually smoked by the COM participants, while EXP participants cited Marlboro as their preferred brand. Differences in baseline smoking topography were observed between EXP and COM participants on measures of average flow rate, t(149) = 3.077, p = .002, and total smoke volume, t(120) = 3.142, p = .002 (see Table 2 for mean values). Baseline group differences were also observed in exposure biomarkers cotinine, t(134) = 2.704, p = .008; hydroxyphenanthrenes, t(148) = 3.385, p < .001; and 1-hydroxypyrene, t(151) = 2.916, p = .004 (see Table 3 for geometric mean values).
Table 1.
Demographic Characteristics of Study Participants in the COM (Buffalo, NY) and EXP (Boston, MA) Groups, 2007
| Variable | COM | EXP |
| n(%) | n (%) | |
| Gender* | ||
| Male | 37 (44.6) | 49 (63.6) |
| Female | 46 (55.4) | 28 (36.4) |
| Race/ethnicity** | ||
| White | 26 (31.3) | 69 (89.6) |
| Black | 47 (56.6) | 3 (3.9) |
| Other | 10 (12.1) | 5 (6.5) |
| Brand usually smoked** | ||
| Newport | 65 (78.3) | 10 (13.7) |
| Marlboro | 16 (19.3) | 39 (53.4) |
| Camel | 2 (2.4) | 24 (32.9) |
| M (SD) | M (SD) | |
| Average age (years)** | 39.6 (9.9) | 28.0 (11.9) |
| Average age began smoking (years) | 17.2 (4.8) | 17.3 (3.0) |
| Years smoking current brand (years)** | 16.7 (9.1) | 6.9 (7.3) |
| Cigarettes/day (n) | 17.0 (8.8) | 17.9 (9.3) |
| Time to first cigarette*** (min)* | 22.3 (33.9) | 35.8 (61.0) |
Note. *p < .05; **p < .001.
Table 2.
Model-Adjusted Mean Smoking Topography Levels by Group and Day
| Topography variable | Day | COM |
EXP |
||
| M | 95% CI | M | 95% CI | ||
| Puff count | 1 | 14.4 | 13.1–15.7 | 13.7 | 12.5–15.0 |
| pgroup = .025 | 4 | 15.2 | 13.7–16.7 | 12.5 | 11.1–13.9 |
| pday = .204 | 15 | 15.0 | 13.5–16.5 | 12.7 | 11.3–14.1 |
| pGroup × Day = .016 | 18 | 14.1 | 12.5–15.6 | 12.3 | 10.9–13.7 |
| Average flow rate (ml/s) | 1 | 36.5 | 33.4–39.7 | 31.5 | 28.5–34.6 |
| pgroup = .014 | 4 | 36.8 | 33.7–40.0 | 31.9 | 28.9–34.9 |
| pday = .243 | 15 | 36.8 | 33.6–39.9 | 32.8 | 29.8–35.7 |
| pGroup × Day = .846 | 18 | 38.0 | 34.7–41.3 | 33.3 | 30.3–36.4 |
| IPI (s) | 1 | 17.6 | 14.8–20.5 | 21.3 | 18.6–24.0 |
| pgroup = .155 | 4 | 19.4 | 15.9–22.9 | 21.6 | 18.2–24.9 |
| pday = .487 | 15 | 18.4 | 15.2–21.7 | 21.7 | 18.6–24.7 |
| pGroup × Day = .693 | 18 | 19.9 | 16.0–23.8 | 21.8 | 18.2–25.3 |
| Duration (s) | 1 | 1.77 | 15.7–19.7 | 1.89 | 17.0–20.8 |
| pgroup = .168 | 4 | 1.65 | 14.6–18.4 | 1.87 | 16.8–20.5 |
| pday = .003 | 15 | 1.72 | 15.1–19.3 | 1.85 | 16.5–20.4 |
| pGroup × Day = .693 | 18 | 1.59 | 14.0–17.9 | 1.76 | 15.8–19.4 |
| Per puff volume (ml) | 1 | 57.5 | 51.8–63.2 | 56.7 | 51.2–62.1 |
| pgroup = .726 | 4 | 55.3 | 49.4–61.2 | 57.1 | 51.5–62.8 |
| pday = .197 | 15 | 53.9 | 47.8–60.0 | 57.9 | 52.3–63.4 |
| pGroup × Day = .145 | 18 | 54.3 | 47.9–60.7 | 54.1 | 48.2–60.0 |
| Total volume (ml) | 1 | 828 | 750–905 | 611 | 539–682 |
| pgroup = .002 | 4 | 836 | 741–931 | 710 | 622–798 |
| pday = .054 | 15 | 798 | 722–874 | 685 | 615–755 |
| pGroup × Day = .027 | 18 | 745 | 660–831 | 648 | 572–724 |
Note. IPI = inter-puff interval; Means adjusted for age, sex, race/ethnicity, and brand. Bolded values are statistically significant.
Table 3.
Model-Adjusted Mean Saliva and Urinary Biomarker Levels by Group and Day
| Biomarker | Day | COM |
EXP |
||
| M | 95% CI | M | 95% CI | ||
| Alveolar CO (ppm) | 1 | 14.1 | 10.6–17.6 | 17.2 | 14.0–20.5 |
| pgroup = .261 | 4 | 14.7 | 11.1–18.3 | 17.5 | 14.2–20.8 |
| pday = .451 | 15 | 13.8 | 10.5–17.2 | 18.1 | 15.0–21.4 |
| pGroup × Day = .679 | 18 | 15.6 | 12.0–19.1 | 18.1 | 14.9–21.2 |
| Geometric mean | 95% CI | Geometric mean | 95% CI | ||
| Cotinine (ng/ml) | 1 | 377 | 282–504 | 239 | 181–315 |
| pgroup = .124 | 15 | 347 | 261–462 | 262 | 197–348 |
| pday = .453 | |||||
| pGroup × Day = .295 | |||||
| 1-Hydroxypyrene (ng/g creatinine) | 4 | 371 | 279–495 | 261 | 201–340 |
| pgroup = .120 | 18 | 381 | 285–510 | 288 | 223–372 |
| pday = .166 | |||||
| pGroup × Day = .496 | |||||
| Naphthols (ng/g creatinine) | 4 | 20,869 | 16628–26196 | 20,869 | 16944–25685 |
| pgroup = .255 | 18 | 20,030 | 16009–25048 | 23,459 | 19273–28544 |
| pday = .074 | |||||
| pGroup × Day = .100 | |||||
| Hydroxyfluorenes (ng/g creatinine) | 4 | 2,996 | 2382–3769 | 2,867 | 2325–3532 |
| pgroup = .672 | 18 | 2,978 | 2369–3747 | 3,165 | 2585–3872 |
| pday = .101 | |||||
| pGroup × Day = .240 | |||||
| Hydroxyphenanthrenes (ng/g creatinine) | 4 | 644 | 527–788 | 555 | 462–666 |
| pgroup = .953 | 18 | 675 | 552–823 | 669 | 563–796 |
| pday = .007 | |||||
| pGroup × Day = .164 | |||||
Note. Means adjusted for age, sex, race/ethnicity, cigarette consumption, brand, and smoking topography. Bolded values are statistically significant.
Self-reported Smoking Behaviors
Perceived Cigarette Self-extinguishment
At baseline, 25.9% of COM participants reported their cigarette self-extinguished “often” or “all the time” while smoking compared with 1.4% of EXP participants, χ2(1) = 18.908, p < .001. At Visit 3, 18.2% of COM participants and 14.5% of EXP participants (who had switched to RIP cigarettes) reported self-extinguishment of their cigarettes, χ2(1) = 0.337, p = 0.562. McNemar’s test showed that the increase in reporting of cigarette self-extinguishment among EXP subjects was statistically significant (p = .012), but the decreased reporting of cigarette self-extinguishment among COM participants was not (p = .180).
Cigarette Consumption
Linear mixed models, which controlled for age, race, gender, and brand, were performed to detect group, time, and interaction effects on the number of cigarettes smoked during the 48-hr field periods (Days 2–3 and Days 16–17). Overall, we observed significant changes in cigarette usage. COM participants smoked 26.6 (SEM 3.3) cigarettes during the first 48-hr period (Days 2 and 3) compared with 30.1 (SEM 3.3) cigarettes during the second (Days 16 and 17). EXP participants smoked 37.7 (SEM 3.2) and 32.6 (SEM 3.1) cigarettes during each of the two 48-hr periods, respectively. The Group × Day interaction was statistically significant (p = .031). The switch to RIP cigarettes among EXP participants appears associated with fewer (14% reduction), rather than more, cigarettes smoked.
Smoking Topography
Linear mixed models, which controlled for age, race, gender, and brand, were performed on measures of smoking topography (puff count, puff velocity, per puff volume, puff duration, inter-puff interval [IPI], and total puff volume). Mean values by group and day are shown in Table 2. No significant effects were observed for IPI. A significant day effect was observed on puff count, with more puffs taken at Day 1 compared with Day 18 (B = 1.5, p = .015), across both groups. Also noted were significant effects of sex (males had lower puff counts, B = −2.1, p < .001), age (decreasing puff counts with increasing age; B = −0.1, p = .024), and brand (BNewport = −3.0 and BMarlboro = −2.4, p’s < .007). There was a significant overall group difference in puff velocity, with higher puff velocity observed among COM participants at all time points (B = 4.7, p = .027). Puff duration showed a significant main effect of day, lower at Day 18 relative to Day 1 (B = 128.7, p = .03) and 4 (B = 105.4, p = .05) across both sites. We also observed substantial sex (B = 285.7, p = .001) and age (B = 8.2, p = .044) effects on puff duration, with males and older smokers taking significantly longer puffs. Per puff volume showed a significant change only at Day 15 compared with Day 18 (B = 3.8, p = .018). Again, significant sex (B = 10.2, p < .001) and age (B = 0.3, p = .018) effects were observed, with males and older smokers taking significantly larger puffs on average. Finally, total puff volume showed a significant Group × Time interaction. Only at Day 1 were the two sites significantly different (B = 120, p = .016); at all other observations, the two sites were parallel in terms of pattern of change. We also observed that, relative to Camel, Newport smokers produced a significantly smaller total puff volume per cigarette (B = 231.2, p < .001). Because the Buffalo participants smoked outdoors, we also examined whether season influenced any of the topography measures in this group and found no statistically significant differences (data not shown).
Exposure Biomarkers
Carbon Monoxide
In a model controlling for age, sex, race, brand, time since last cigarette smoked (dichotomized as ≤30 min vs. ≥31 min), and smoking topography (total puff volume, puff velocity, and IPI), we found no significant overall effect of site (p = .261), no significant overall effect of day (p = .451), and no Group × Day interaction (p = .679), indicating that the patterns of change in exhaled CO did not differ between the sites or change over time. Mean values and confidence intervals are shown in Table 3. We did observe a significant main effect of age (older smokers showed higher average CO, p = .046) and of brand (Marlboro smokers significantly higher than Camel smokers, p < .001).
Cotinine
In a model controlling for age, sex, race, brand, total cigarettes smoked the previous day, and smoking topography, cotinine did not vary according to group or time nor did these factors interact (see Table 3). As expected, the number of cigarettes smoked the previous day was significantly associated with cotinine level (p < .001). Analysis of cotinine levels by race revealed no significant effects (pWhite vs. other = 0.252, pBlack vs. other = 0.184). We observed a borderline significant effect of gender (p = .052), with males showing somewhat higher cotinine.
PAH Metabolites
In models controlling for age, sex, race, brand, total cigarettes smoked in the last 48 hr, and smoking topography, various patterns of change were observed among the PAH metabolite biomarkers. These are illustrated in the model-adjusted means by group and day in Table 3. For 1-OH-PYR, we observed no significant or borderline effects of group, day, or their interaction. A significant effect of race was noted, with Black participants showing lower 1-OH-PYR levels relative to “other” participants (p = .029). Cigarette consumption was weakly related to increased 1-OH-PYR levels (p = .055). A borderline significant Group × Time interaction was observed for naphthols, with smokers from the EXP group showing an approximate increase of 12.4% compared with a 4% decrease among Buffalo participants. We observed significant main effects of brand, with Marlboro (p = .002) smokers showing higher overall naphthol levels relative to Camel smokers. Higher age (p = .019) and cigarette consumption in the last 48 hr (p = .013) were both independently associated with higher naphthol levels. Hydroxyfluorenes showed a mixed pattern of associations. A borderline effect of time was observed, with levels increasing 10.4% in the EXP group, while essentially unchanging in the COM group. Age (p = .005) and cigarette consumption (p = 0.046) were associated with higher hydroxyfluorene levels. Brand was also a significant independent covariate, with Newport (p = .024) and Marlboro (p = .002) both showing higher hydroxyfluorene levels relative to Camel smokers. Finally, a significant effect of time was observed for hydroxyphenanthrene, with EXP participants showing a 20.5% increase across visits relative to a 4.8% increase among COM participants. Black participants, relative to other participants, showed lower average hydroxyphenanthrene levels (p = .034). Age (p = .007) and cigarette consumption (p = .001) were also independently associated with higher hydroxyphenanthrene levels.
We also ran a parallel set of analyses on the EXP group only, using repeated measures analysis of variance to examine changes in smoking topography and exposure biomarkers before and after the switch. Across all dependent variables of interest, results reflected the findings from the mixed model analyses (data not shown).
Discussion
This study aimed to determine whether short-term switching from conventional to RIP cigarettes is associated with changes in smoking behavior and/or exposure to smoke constituents that suggest increased risk to the smoker. No changes in smoking topography attributable to the RIP design modification were identified in models adjusted for a broad range of potential confounding variables. Switching to RIP-compliant cigarettes did not appear to significantly affect subjects’ smoking behaviors. This disconfirms the hypothesis that smokers using RIP cigarettes would take more frequent puffs, or puff more intensively, in response to the self-extinguishing feature. Indeed, more EXP smokers noticed self-extinguishment after switching to RIP cigarettes, and the number reporting self-extinguishment became proportionately similar to those reported by RIP-experienced COM smokers and in previous studies (O’Connor et al., 2006). Additionally, those who did notice self-extinguishment did not demonstrate a change in smoking topography following the switch to RIP cigarettes.
The finding of generally similar exposures before and after the product switch is consistent with a study done in Canada examining yield-in-use from cigarette filters (Côté, Létourneau, Mullard, & Voisine, 2010). While some markers of PAH exposure were elevated among the EXP smokers after switching, these changes were not consistent across biomarkers. A biomarker of exposure to phenanthrene, an International Agency for Research on Cancer Group 3 (not classifiable as to its carcinogenicity to humans) compound, was substantially (20.5%, pday = .007) elevated by switching to RIP cigarettes in this study. Interestingly, smoke machine yields of phenanthrene were found to be no higher in RIP cigarettes by Connolly et al. (2005), except within the Newport brand, where a 20% increase was observed. However, the observed increases in hydroxyphenanthrenes within the EXP group were consistent across brands, so the effect does not appear driven by brand-specific changes. Conversely, although machine measured smoke yields of CO were higher in RIP versions of popular brands (Connolly et al.), no increase in exhaled CO among EXP smokers was observed. The toxicological significance of these observations is unclear. In vitro toxicology data, for example, indicate no difference in genotoxicity (Ames assay and sister chromatid exchanges) or cytotoxicity (neutral red assay) for cigarette smoke derived from banded paper technologies compared with standard paper (Theophilus et al., 2007). Similarly, in vivo inhalation and dermal tumor promotion studies in mice showed no significant differences between banded and standard papers (Theophilus et al.).
The baseline PAH biomarker levels among smokers in the current study are substantially higher than levels reported among a nationally representative sample of smokers reported in the National Health and Nutrition Examination Survey (Li et al., 2008). However, Li et al. (2008) defined smoking status as serum cotinine greater than 15 ng/ml, rather than by self-report, and may therefore include more low-level and nondaily smokers. In contrast, nondaily smokers were excluded from the current study. Observed levels of naphthols in the current study are similar to those reported by Benowitz et al. (2005) during use of regular and light cigarettes.
This study is subject to a number of limitations. First, the study populations at the two sites were demographically different. This was addressed in part by modeling adjusting for age, sex, and race and by employing a repeated measures design such that participants served as their own controls. Future research studies should consider matching participants across sites to reduce the potential for confounding. Second, we examined changes associated only with leading cigarette brands (Marlboro, Newport, and Camel), all of which have certified their compliance with NY State ignition propensity standards and are believed to use similar banding technologies to achieve compliance (Alpert et al., 2010; Connolly et al., 2005). However, less is known about designs used by smaller manufacturers of discounted cigarette brands to comply with regulations. Third, this study intentionally represents a short-term switching design and so the longer term effects of RIP cigarettes on biomarkers of exposure cannot be estimated. We acknowledge that practicality issues experienced by the two sites may have introduced some confounds into the topography data, such as posture (standing vs. sitting), and seasonal impacts on smoking urgency for those participants (in Buffalo) smoking outdoors. However, because we were primarily interested in relative effects, we feel that the overall impact of such confounds is minimal. Finally, because the differences between RIP and non-RIP products are difficult to determine after smoking, we were unable to objectively verify compliance. However, we have little reason to suspect that substantial levels of deviation from protocol occurred.
The present findings have relevance as the Food and Drug Administration begins to create procedures for regulating tobacco products. We found that a relatively small alteration in cigarette design increased exposure to some constituents while not affecting others. Small alterations to existing products are likely not unique—cigarette manufacturers routinely refine their products while in the market (Wayne & Connolly, 2009), and presumably, these changes in design may be reflected in changes to emissions profiles. This points to the need for vigilant monitoring of both products and users of those products and also for careful consideration of what constitutes a “substantially equivalent” tobacco product.
This study suggests that small increases in exposure to phenanthrene among smokers switched to RIP versions of their usual cigarette brands might occur. The toxicological implications of such differences, if any, are unknown. At the same time, exposures to nicotine, CO, and other PAHs were not significantly affected. Jurisdictions that have implemented or are considering RIP regulations should remain primarily focused on reduced fire risk as the primary outcome but should consider monitoring the changes to cigarette design and smoke emissions that accompany compliance with the regulation as part of routine surveillance activities.
Funding
This work was supported by a grant from the National Cancer Institute at the National Institutes of Health (R01CA117108 to RJO) and by a grant from the Roswell Park Alliance Foundation.
Declaration of Interests
KMC has testified on behalf of plaintiffs in lawsuits against the tobacco industry.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services. The use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention.
References
- Alpert HR, O'Connor RJ, Spalletta R, Connolly GN. Recent advances in cigarette ignition propensity research and development. Fire Technology. 2010;46:275–289. doi: 10.1007/s10694-008-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott D, Berteletti F. Europe: Agreement on reducing cigarette fires. Tobacco Control. 2008;17:4–5. [PubMed] [Google Scholar]
- American Society for Testing and Materials. ASTM E2187-04 Standard Test Method for Measuring the Ignition Strength of Cigarettes. 2004. Retrieved from American Society for Testing and Materials website, http://www.astm.org/Standards/E2187.htm. [Google Scholar]
- Bavley A. Smokers hot over fire-safe cigarettes. 2009, August 7. Retrieved from The Kansas City Star website, http://cms.firehouse.com/web/online/News/Smokers-Hot-Over-Fire-Safe-Cigarettes/46$64857. [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Bernert JT, Wilson M, Wang L, Allen F, et al. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1376–1383. doi: 10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Coalition for Fire-Safe Cigarettes. Legislative adoptions. 2010a. Retrieved from http://www.firesafecigarettes.org/categoryList.asp?categoryID=77&URL=Legislative%20updates/Adoptions. [Google Scholar]
- Coalition for Fire-Safe Cigarettes. Model legislation. 2010b. Retrieved from http://www.firesafecigarettes.org/assets/files//FSCmodel07.pdf. [Google Scholar]
- Connolly GN, Alpert HR, Rees V, Carpenter C, Wayne GF, Vallone D, et al. Effect of the New York State cigarette fire safety standard on ignition propensity, smoke constituents, and the consumer market. Tobacco Control. 2005;14:321–327. doi: 10.1136/tc.2005.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté F, Létourneau C, Mullard G, Voisine R. Estimation of nicotine and tar yields from human-smoked cigarettes before and after the implementation of the cigarette ignition propensity regulations in Canada. Regulatory Toxicology and Pharmacology. 2010 doi: 10.1016/j.yrtph.2010.03.004. Epub ahead of print. March 18; PMID: 20303374. [DOI] [PubMed] [Google Scholar]
- Fire safety standards for cigarettes. ID No. DOS-53-02-00018-RP. NYS Register. 3 September 2003. [Google Scholar]
- Goswami H. Reduced ignition propensity cigarettes—Facts, fiction, and manipulations. 2007, November. [PowerPoint Slides]. Presented at 6th Annual Meeting of the International Society for Prevention of Tobacco Induced Diseases, Little Rock AR. Retrieved from http://www.slideshare.net/burningbrain/reduced-ignition-propensity-cigarettes-facts-fiction-and-manipulations. [Google Scholar]
- Gunja M, Wayne GF, Landman A, Connolly G, McGuire A. The case for fire safe cigarettes made through industry documents. Tobacco Control. 2002;11:346–353. doi: 10.1136/tc.11.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR., Jr . The smoking-material fire problem. 2008. Retrieved from National Fire Protection Association http://www.nfpa.org/assets/files//PDF/OS.Smoking.pdf. [Google Scholar]
- Harris JE. Incomplete compensation does not imply reduced harm: Yields of 40 smoke toxicants per milligram nicotine in regular filter versus low-tar cigarettes in the 1999 Massachusetts Benchmark Study. Nicotine & Tobacco Research. 2004;6:797–807. doi: 10.1080/1462220042000274266. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hanson K, Briggs A, Parascandola M, Genkinger JM, O'Connor R, et al. Clinical trials methods for evaluation of potential reduced exposure products. Cancer Epidemiology Biomarkers & Prevention. 2009;18:3143–3195. doi: 10.1158/1055-9965.EPI-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karter MJ, Kissinger TL, Miller AL, Harwood B, Fahy RF, Hall JR. Cigarette characteristics, smoker characteristics, and the relationship to cigarette fires. Fire Technology. 1994;30:400–431. [Google Scholar]
- Li Z, Romanoff LC, Hussain N, Trinidad DA, Porter EN, Jones RS, et al. Measurement of urinary mono-hydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and isotope dilution gas chromatography/high resolution mass spectrometry. Analytical Chemistry. 2006;78:5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environmental Research. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- McGuirk R. Australia wants to fast track fire-safe cigarettes. 2009, February. Retrieved from Associated Press http://abcnews.go.com/International/wireStory?id=6918757. [Google Scholar]
- National Cancer Institute. Risks associated with smoking cigarettes having low machine-measured yields of tar and nicotine. Bethesda, MD: Author; 2001. Smoking and Tobacco Control Monograph 13. [Google Scholar]
- O’Connor RJ, Giovino G, Fix B, Hyland A, Hammond D, Fong GT, et al. Smokers’ reactions to reduced ignition propensity cigarettes. Tobacco Control. 2006;15:45–49. doi: 10.1136/tc.2005.013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwick R. Canada gets its house in order. Injury Prevention. 2005;11:259–260. doi: 10.1136/ip.2005.010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theophilus EH, Pence DH, Meckley DR, Keith Shreve W, Ayres PH, Bombick BR, et al. Toxicological evaluation of cigarettes with two banded cigarette paper technologies. Experimental Toxicology & Pathology. 2007;59:17–27. doi: 10.1016/j.etp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Tobacco Control Programme. Overview Of Responses To Regulatory Proposal For Reducing Fire Risks From Cigarettes. 2002. Retrieved, from Health Canada http://www.hc-sc.gc.ca/hl-vs/pubs/tobac-tabac/orrp-arpr/index-eng.php. [Google Scholar]
- Warwick WR. 2000. Consumer contacts concerning Merit-banded paper. Retrieved from http://www.pmdocs.com (Bates Number 2078190899) [Google Scholar]
- Wayne GF, Connolly GN. Regulatory assessment of brand changes in the commercial tobacco product market. Tobacco Control. 2009;18:302–309. doi: 10.1136/tc.2009.030502. [DOI] [PubMed] [Google Scholar]