Table 2.
Scope of Rh-Catalyzed Hydroamination of Primary Aminesa
| entry | mol % [Rh] | aminoalkene | product | time (h) | yieldb (dr)c | ratio (amine:imine)c | |
|---|---|---|---|---|---|---|---|
| 1 | 3 |
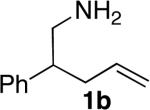
|
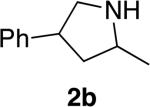
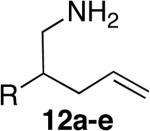
|
15 | 66% (dr = 1.3:1) | 9:1 | |
| 2d | 4 |
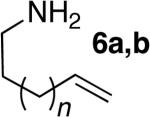
|
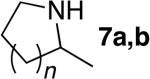
|
7 | a; n=1; 76%e | >95:5 | |
| 3d | 4 | 8 | b; n=2; 77% | >95:5 | |||
| 4f,g | 18 |
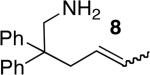
|
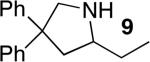
|
15 | 40% | >95:5 | |
| 5f,h | 3 |
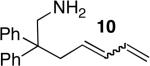
|
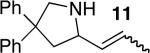
|
14 | 64%i | 7:1 | |
| 6j | 3 |
|
a; R=OTBDPS |
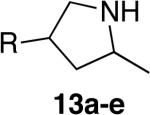
|
18 | 71% (dr = 1.6:1) | >95:5 |
| 7 | 3 | b; R=4-(CO2Me)C6H4 | 21 | 64% (dr = 1.6:1) | 10:1 | ||
| 8 | 5 | c; R=4-(CH2CN)C6H4 | 12 | 79% (dr = 1.5:1) | >95:5 | ||
| 9 | 5 | d; R=4-(CH3(O)C)C6H4 | 16 | 61% (dr = 1.5:1) | 10:1 | ||
| 10 | 5 | e; R=4-(CH2OH)C6H4 | 8 | 56%k (dr = 1.1:1) | 7:1 | ||
|
|
||||||
| 11 | 1 | a; R = H | a; R = H | 5 | 87% | >95:5 | |
| 12 | 2 | b; R = Cl | b; R = Cl | 6 | 84% | >95:5 | |
| 13 | 1 |
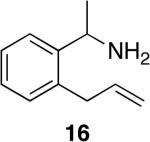
|
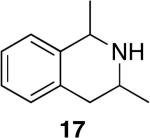
|
7 | 74% dr = (1.1:1) | >95:5 |
Reaction conditions: 0.5 mmol aminoalkene, [Rh(CH3CN)2COD]BF4 (1-18 mol %), and L1 (1.2-19 mol %) in 1 mL of tBuOH at 70 °C for unless otherwise specified.
Isolated yield.
Ratio determned by 1H NMR spectroscopy.
Product isolated as Boc carbamate.
NMR yield; the product was isolated in 67% yield as an 10:1:1 mixture of 7a-Boc:BocNEt2:N-Boc-1-amino-3-pentene
Reaction run at 100 °C in dioxane.
3.3:1 mixutre of E:Z isomers.
10:1 mixture of E:Z isomers.
Isolated as a 1.8:1 mixture of olefin isomers (internal:terminal).
Reaction run on a 0.25 mmol scale.
Isolated as a 97:3 mixture of alcohol:aldehyde products.
