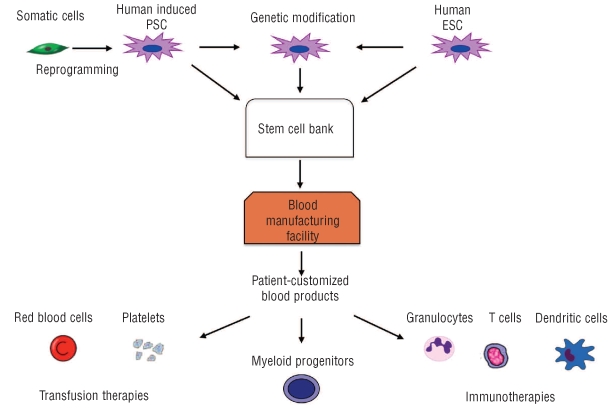
Figure 1.
The use of human induced pluripotent stem cells (PSC) and human embryonic stem cells for manufacturing blood products. Human PSC lines with the most commonly used RBC and platelet genotypes/phenotypes can be obtained and used for continuous manufacturing of blood products. To meet specific clinical needs (e.g., transfusion of alloimmunized patients, patients with rare blood groups), cells with unique genotypes/phenotypes can be obtained from a stem cell bank to manufacture blood products on demand. In addition, human PSC can be genetically modified to produce RBC for drug delivery or platelets for targeting specific pathways in the coagulation cascade to treat bleeding disorders or coagulopathies. Human PSC-derived hematopoietic progenitors can be used to treat bone marrow failure due to chemotherapy-induced myelotoxicity. Human PSC also provide an opportunity to manufacture dendritic cell-based vaccines for cancer immunotherapy with a broad variety of MHC genotypes/phenotypes. By using human PSC genetically engineered to express tumor-specific T-cell receptor, cytotoxic T cells can be produced to target malignant tumors.