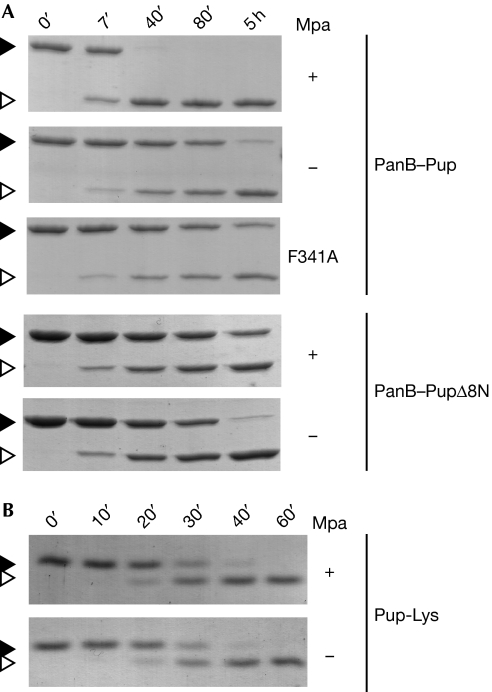
Figure 4.
Mpa enhances the depupylation of proteasomal substrates in vitro. (A) The depupylation of PanB–Pup (3 μM) by Dop (0.5 μM) is accelerated by Mpa (0.2 μM) in the presence of ATP as analysed by SDS–PAGE and Coomassie staining. By contrast, the depupylation of PanB–PupΔ8N (3 μM; Pup is truncated by the first eight amino acids) is not accelerated by the presence of Mpa and a translocation-defective Mpa variant (F341A) cannot enhance depupylation of PanB–Pup. Black arrowhead, PanB–Pup; white arrowhead, PanB. (B) The presence of Mpa (0.2 μM) does not affect the rate of Pup-Lys depupylation. Reaction described in (A) using Pup modified with lysine (Pup-Lys; 10 μM) as substrate. Black arrowhead, Pup-Lys; white arrowhead, Pup. Dop, deamidase of Pup; Mpa; mycobacterial proteasomal ATPase; Pup, prokaryotic ubiquitin-like protein; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis.