Summary
We investigated the responses of single neurons in primary visual cortex (area V1) of awake monkeys to chromatic stimuli. Chromatic tuning properties, determined for homogeneous color patches presented on a neutral gray background, varied strongly between cells. The continuum of preferred chromaticities and tuning widths indicated a distributed representation of color signals in V1. When stimuli were presented on colored backgrounds, chromatic tuning was different in most neurons, and the changes in tuning were consistent with some degree of sensitivity of the neurons to the chromatic contrast between stimulus and background. Quantitatively, the average response changes matched the magnitudes of color induction effects measured in human subjects under corresponding stimulus conditions.
Introduction
In color vision, probably more so than in any other visual modality, the relation between the sensory input and the percept eludes a simple explanation. For example, our percepts of pure colors, or unique hues, do not correspond to the responses of single cone types, nor to a simple combination of them (Valberg, 2001). Furthermore, stimuli of the same chromaticity may evoke different color percepts, depending on the visual context (Land, 1959; Wesner and Shevell, 1992; Brown and MacLeod, 1997; Kraft and Brainard, 1999). The mechanisms underlying these contextual influences are not well understood. The lack of a simple relationship between stimulus and percept indicates that considerable processing of the photoreceptor signals must take place to yield the neural activity that we experience as a color percept. While the first steps of color processing in the retina have been intensively studied and some degree of understanding has been achieved (for review, see Martin, 1998; Nathans, 1999; Dacey, 2000), less is known about the representation and processing of chromatic stimuli in the cortex.
Chromatic input to the visual cortex from the lateral geniculate nucleus (LGN) is encoded in an opponent fashion (De Valois et al., 1966; Wiesel and Hubel, 1966) that matches the color axes defined by cone opponency (Derrington et al., 1984). Color opponent cells are also found in the primary visual cortex (area V1) (Motokawa et al., 1962; Gouras, 1974; Poggio et al., 1975; Michael, 1978a; Livingstone and Hubel, 1984; Ts’o and Gilbert, 1988; Conway, 2001), but chromatic tuning properties are much more diverse than in LGN cells (Lennie et al., 1990; De Valois et al., 2000; Hanazawa et al., 2000).
One of the most pronounced properties of color vision is the dependence of the perceived color of a stimulus on the visual context in which it is presented. Consequently, one would expect to find such contextual influences in the neuronal activity that represents the color percept. Context-dependent responses to color stimuli have been reported for cortical visual area V4 (Zeki, 1983b; Schein and Desimone, 1990), but not for V1 (Zeki, 1983a). Recently, there has been an increasing number of studies on nonclassical receptive-field influences in V1 for different visual modalities (Knierim and Van Essen, 1992; Sillito and Jones, 1996), including the brightness of achromatic stimuli (Rossi and Paradiso, 1999; Kinoshita and Komatsu, 2001). For color stimuli, such effects have been reported in the context of figure-ground segregation (Zipser et al., 1996). It is, however, not clear to what extent they may be relevant for color appearance. We therefore investigated the responses of V1 neurons to stimuli presented in different chromatic contexts. We used homogeneously colored square patches, similar to stimuli frequently employed in psychophysical experiments on color vision. Consequently, in contrast to many other studies of V1 neurons, we recorded mainly from cells that responded strongly to such stimuli of relatively low spatial frequency. We did not attempt to use each cell’s preferred stimulus geometry, but rather investigated the responses of the cells to the same types of stimuli. We measured chromatic tuning properties, quantified the contextual influences, and compared them with the strength of induction effects observed in human color perception.
Results
Tuning Properties
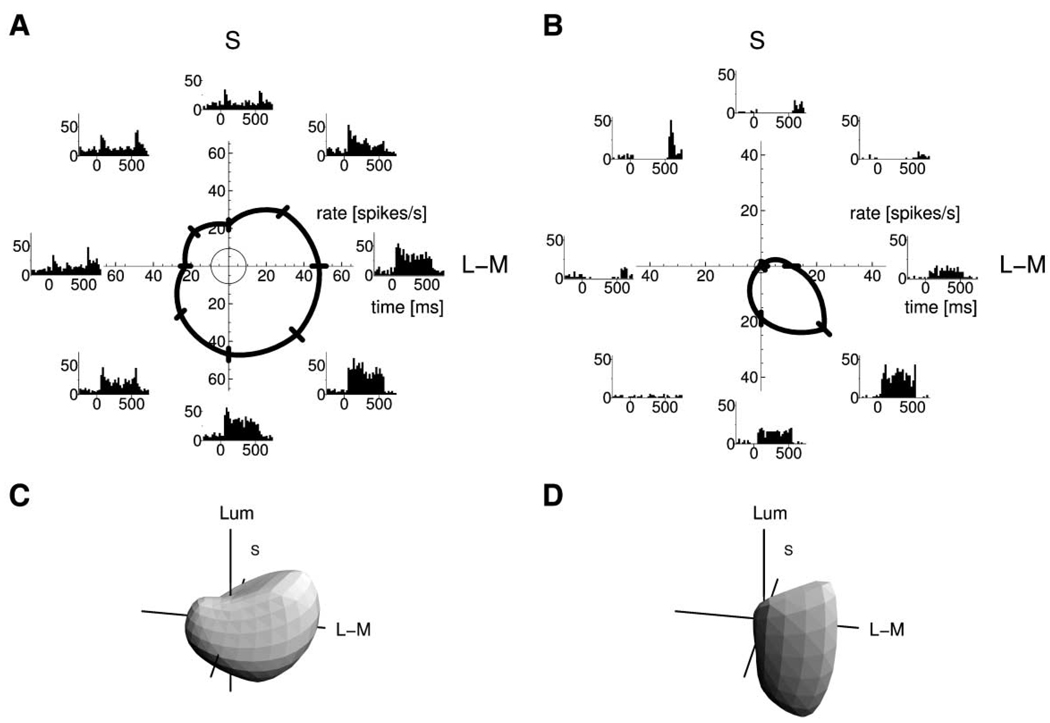
Figure 1 shows responses of two neurons to illustrate different tuning characteristics. The neuron on the left is tuned broadly in the (L-M,S) plane (top graph), and it responds to all hues, but stronger to some than to others. The neuron on the right shows opponency, being excited by some hues and inhibited by others. It has a narrower tuning in the (L-M,S) plane. As the plots on the bottom show, tuning width along a vertical meridian in three-dimensional color space, i.e., for brighter and darker stimuli, cannot be inferred from the tuning width for isoluminant stimuli. The neuron on the left has a relatively broader tuning in azimuth than in elevation, while the opposite is the case for the neuron on the right.
Figure 1. Chromatic Tuning of Two V1 Neurons.
(A and B) Tuning to isoluminant stimuli. Horizontal axis, L-M cone contrast variation; vertical axis, S cone contrast variation. Stimulus hue changes as a function of polar angle. Data points in the polar plots show mean spike rate in a time window 50–150 ms after stimulus onset, error bars denote standard error. Tuning curves are linear interpolations of data points. Thin circles around the centers of polar plots indicate spontaneous firing rates. Insets show peri-stimulus time histograms for responses to stimuli in the corresponding directions.
(C and D) Tuning contours in three-dimensional color space, derived from responses to stimuli in 26 directions.
(C) Broadly tuned, nonopponent neuron.
(D) Color-opponent neuron tuned narrowly to different hues (azimuth) and more broadly tuned to stimuli with luminance differences (elevation).
In the following, we consider tuning in the (L-M,S) plane. To quantify tuning properties, we fit a circular normal function, fc = A0 + A exp((cos(ϕ − ϕ0) − 1)/σ2), to the responses to stimuli in different chromatic directions ϕ in the (L-M,S) plane. For most tuning curves, this method yielded good fits (χ2, 4 d.f., p < 0.15). The tuning curves that did not give good fits (n = 5) were bimodal and therefore could not be described well by a circular normal function. In these cases, the function was fitted to the highest peak of the tuning curve. The fitting procedure served to interpolate the data points by a smooth function to derive tuning parameters like tuning peak direction and tuning width. Using other interpolation functions, like powers of cosine functions or polynomials, yielded similar results.
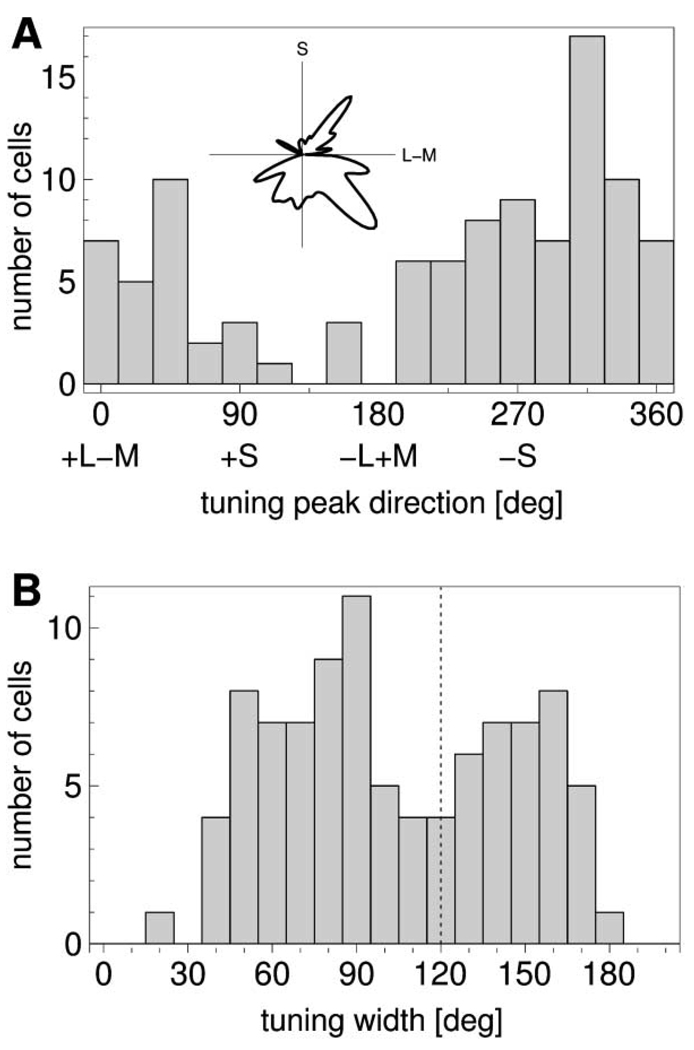
Figure 2A shows the distribution of tuning peaks as a function of chromatic direction. In accordance with the findings of other studies (Lennie et al., 1990; De Valois et al., 2000; Hanazawa et al., 2000), the distribution is continuous, but it is not uniform. Furthermore, the distribution is highly asymmetric. The peaks in the distribution do not coincide with the coordinate axes, as for cells in the LGN (Derrington et al., 1984). The main peak of the distribution lies in the fourth quadrant of the (L-M,S) plane, corresponding to the yellow/orange region. There is also a relatively high number of tuning directions in the third quadrant, corresponding to greenish colors. Other distinct peaks are apparent for blue (second quadrant), as well as for purple in the first quadrant (Dow and Vautin, 1987). The coordinate axes were the directions that tended to have the lowest incidence of tuning peaks.
Figure 2. Chromatic Tuning for Isoluminant Colors.
Tuning parameters were derived from interpolated responses in the (L-M,S) plane (see text).
(A) Distribution of tuning peak directions. The inset shows a polar plot of the same data, smoothed by replacing each data point with a Gaussian of 5° width. The distribution is continuous, nonuniform, and asymmetric.
(B) Distribution of tuning curve widths (full width at half height). The dashed line denotes the width of a cosine function, corresponding to linear combination of cone inputs. The distribution indicates that most neurons combine cone inputs nonlinearly.
The distribution of tuning widths (Figure 2B) is broad and appears bimodal. The peaks of the distribution lie below and above the width of a cosine function, respectively. This indicates nonlinear processing of cone inputs, in agreement with the findings by De Valois et al. (2000).
Effects of Background
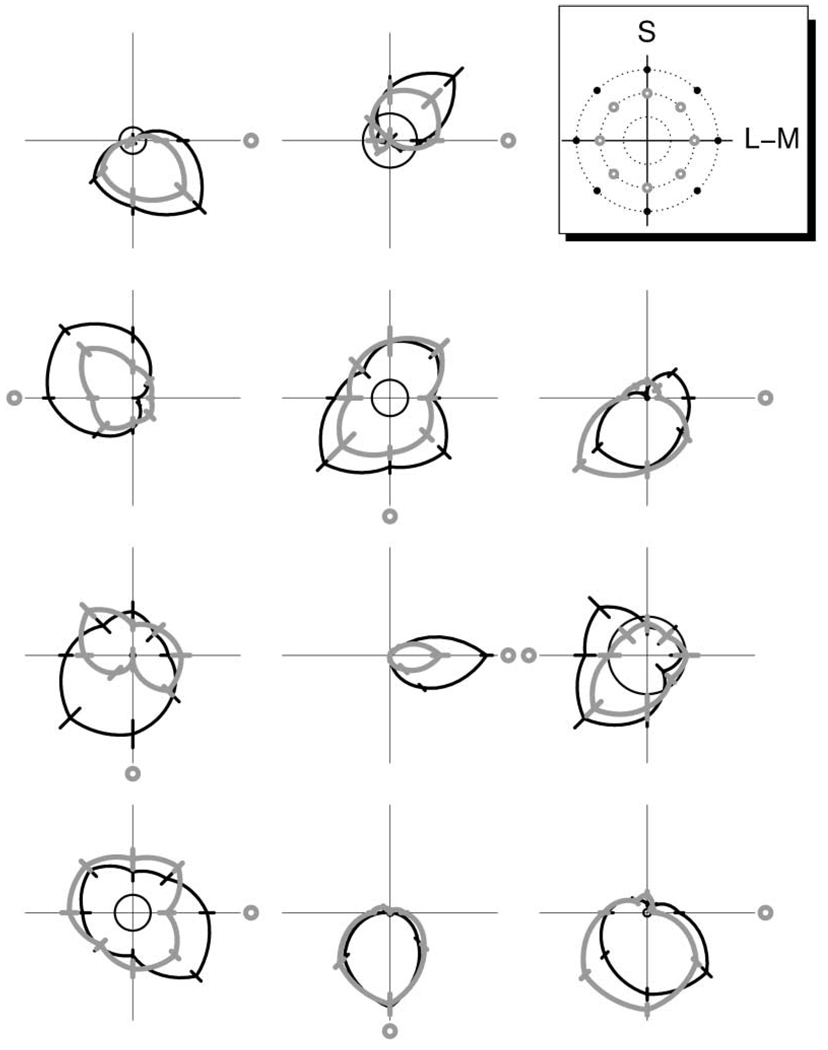
In the following, we maintain the term “stimulus” to describe the single color patch stimulating the receptive field of a neuron. To investigate effects of background chromaticity, for each trial it was chosen randomly whether background chromaticity remained neutral gray throughout the trial or changed for the duration of the stimulus presentation. With respect to the gray background, chromatic backgrounds had a cone contrast of 0.1, while stimuli had a cone contrast of 0.15. Tuning curves of 11 neurons for isoluminant stimuli on the gray background and on a chromatic background, respectively, are shown in Figure 3. We compared chromatic tuning under the two background conditions by testing for differences between responses to the same stimuli. In 72 out of 94 cells (77%), responses to at least one of the stimuli were significantly different (t test, p < 0.01) when presented on the colored background as compared to presentation on the neutral gray background.
Figure 3. Effect of Background Chromaticity on Tuning Curves.
Tuning curve pairs of eleven neurons for stimuli on gray background (black curves) and on colored background (gray curves), respectively. Coordinate axes as in Figure 1 (A and B). Inset at top right indicates the chromaticities of the eight stimuli (black dots) and the eight possible background chromaticities (gray circles) in the isoluminant plane. In each plot, a small gray circle indicates the direction of the chromaticity of the colored background. For each neuron, both tuning curves were obtained during the same experiment, in which the combination of stimulus and background color was chosen randomly from trial to trial. For most neurons, a response reduction was observed with chromatic background for stimuli in or near the direction of the background chromaticity. Several neurons showed a response enhancement for stimuli near the opposite direction.
The most common type of difference (51 cells, 71%) was a reduction in response magnitude for stimuli in the chromatic direction of the background color or the neighboring directions. In approximately half of the cells with altered tuning (37, 51%), responses to stimuli with directions roughly opposite to the background color were enhanced.
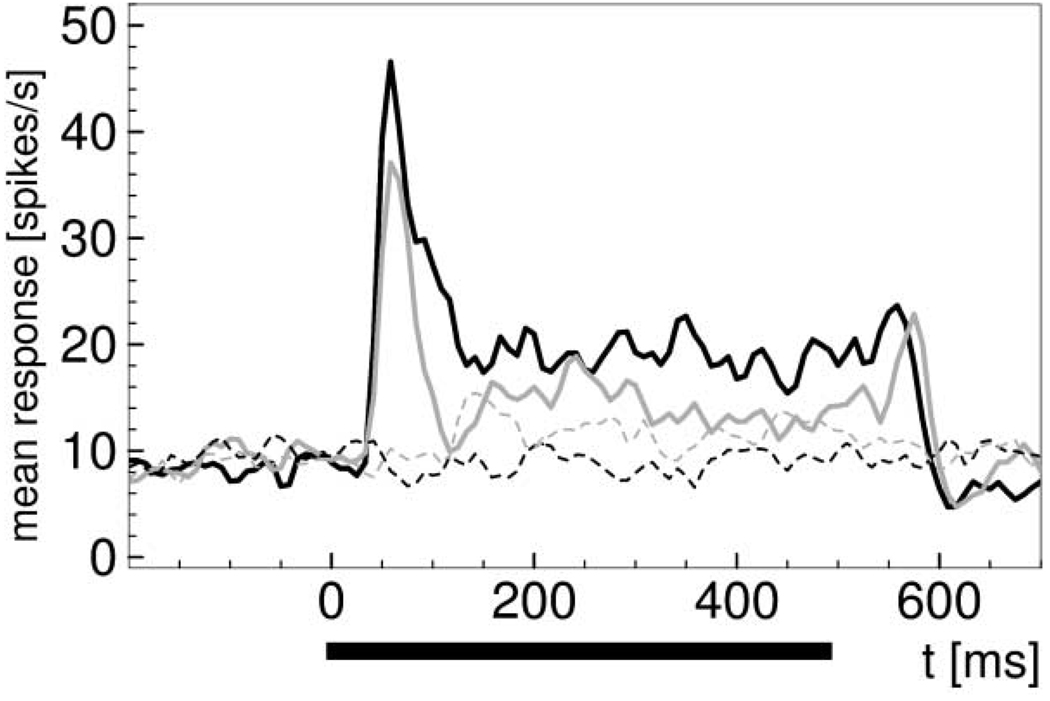
The influence of the background typically appeared early in the time course of the responses (Knierim and Van Essen, 1992; Li et al., 2000), as illustrated in Figure 4. It was strongest around 100 ms after stimulus onset, slightly later than for line stimuli as reported by Li et al. (2000). In control conditions where the background changed without a stimulus being presented, i.e., the chromaticity in the region of the receptive field remained gray, direct responses to the background change, with latencies of more than 100 ms (Li et al., 2000), were observed in 18 cells (19%).
Figure 4. Time Course of Responses.
Average responses of 23 cells that had been tested with stimuli on gray and on (+L-M) backgrounds. Solid lines show mean spike rate to (+L-M) stimuli presented for 500 ms. Dashed lines show activity for trials without stimulus, i.e., the stimulus region remained gray during the trial. Black line, gray background; gray line, (+L-M) background. Responses to stimuli on colored background are lower than to stimuli on gray background.
Differences in responses to the same stimuli on different backgrounds suggest that responses are determined not only by the chromaticity of the stimulus, but also to some degree by the chromatic contrast of the stimulus to the background. When a stimulus of a certain chromatic direction (hue) is presented on a background of the same chromatic direction (but weaker saturation), its chromatic contrast is reduced as compared with presentation on the gray background. Correspondingly, in most neurons the response is reduced. To quantify this influence of the background on the responses to color stimuli, we compared the reduction in the neurons’ responses with the reduction in stimulus contrast. We calculated a background modulation index, which measures the difference in responses r to stimuli in the direction of the background as a fraction of the difference in cone contrast c between stimulus and background,
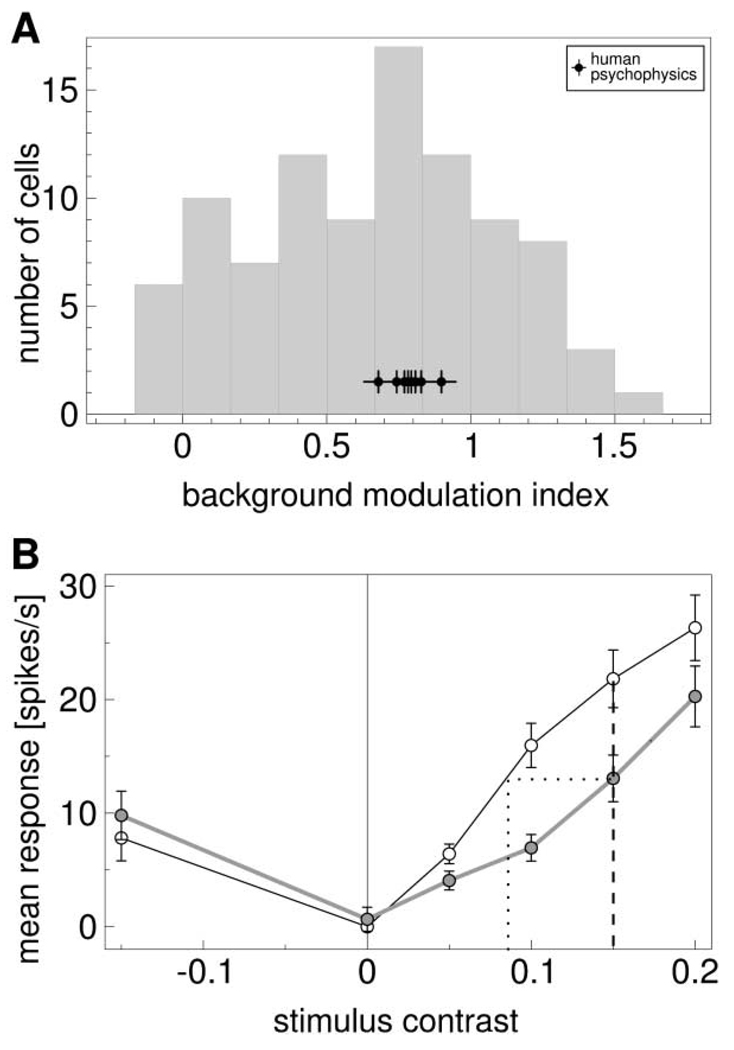
rc and cc are response and cone contrast for the stimulus on the chromatic background, respectively, and r0 and c0 are for the stimulus on the gray background, respectively. An index of zero indicates that the neuron is insensitive to background chromaticity, giving same responses for same stimuli presented on different backgrounds. An index of 1 indicates that the reduction of the response matches the reduction of the contrast between stimulus and background. A negative index occurs if the response to the stimulus on the colored background is stronger than the response to the stimulus on the gray background. Figure 5A shows the distribution of this index. The values are broadly distributed, but most values are above zero, indicating a modulating effect of the background. The median of the distribution of 0.69 indicates that this modulation is on average not quite as strong as one would expect if the responses would represent the contrast of the stimulus to the background.
Figure 5. Effect of Background Chromaticity on Response Strength.
(A) Distribution of background modulation index, measuring the reduction of responses to stimuli on a chromatic background relative to the responses for the same stimuli on the neutral gray background. Stimuli had a cone contrast of 0.15 in the same direction as the chromatic background with contrast 0.1. The median of the distribution is 0.69. Black data points with crosses indicate corresponding values for psychophysical data of eight subjects (median: 0.79).
(B) Mean response as a function of stimulus contrast. Stimuli were from an axis in the (L-M,S) plane in the direction of the chromatic background color. Zero stimulus contrast corresponds to a gray stimulus. Negative contrast corresponds to a stimulus chromaticity opponent to the chromatic background. Black, responses to stimuli on gray background; gray, responses to stimuli on chromatic background. Background contrast was 0.10. Data are means of 61 cells that had been tested with stimuli of different contrasts. For chromatic backgrounds, responses were reduced. The response for stimuli with a contrast of 0.15 (vertical dashed line) on a chromatic background corresponded to the response to a stimulus with lower contrast on a neutral background (dotted lines). Conversely, stimulus contrast needs to be increased on chromatic backgrounds to elicit the same response as on a neutral background.
A complementary view of the background influence is to consider the response differences as a difference in signaled stimulus contrast. Figure 5B shows mean responses as a function of stimulus contrast for both background conditions. The response in the chromatic background condition for our standard stimulus contrast of 0.15 (dashed vertical line) is of a magnitude that would indicate a stimulus of lower contrast (0.085) in the neutral background condition (dotted lines). The response difference corresponds to a contrast reduction of 0.065. Thus, the same stimuli may lead to different responses, whereas different stimuli may elicit the same responses, depending on chromatic context.
Effects of Remote Colors
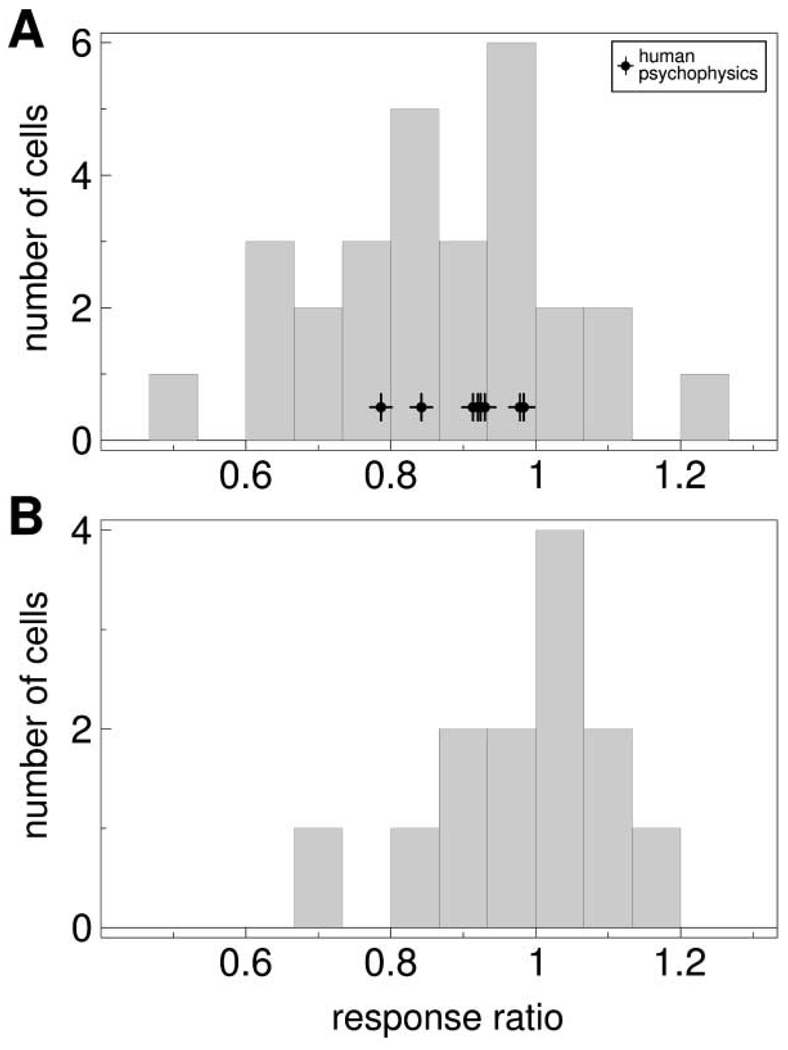
To test lateral interactions besides the immediate spatial contrast of the stimulus, we presented, in addition to the color stimuli at the receptive field position, 2° color patches at a distance of 4° to 6° from the receptive field. The response to the stimulus was often different when these remote fields were present (Figure 6). We quantified this difference by the ratio of the response to the stimulus in the presence of the remote fields, rrem, to the response in the absence of the remote fields, r0: ρrem = rrem/r0. Figure 7A shows the distribution of response ratios ρrem for remote fields of the same chromaticity as the stimulus. The distribution is shifted to values lower than 1 (p < 0.001), with a median of 0.88, indicating an average reduction of the response when the remote fields were present. In contrast, the distribution for the responses when remote fields had the opponent color of the stimulus (Figure 7B) shows no significant shift. This indicates that, like the effect of the background, the influence of remote color patches is color specific.
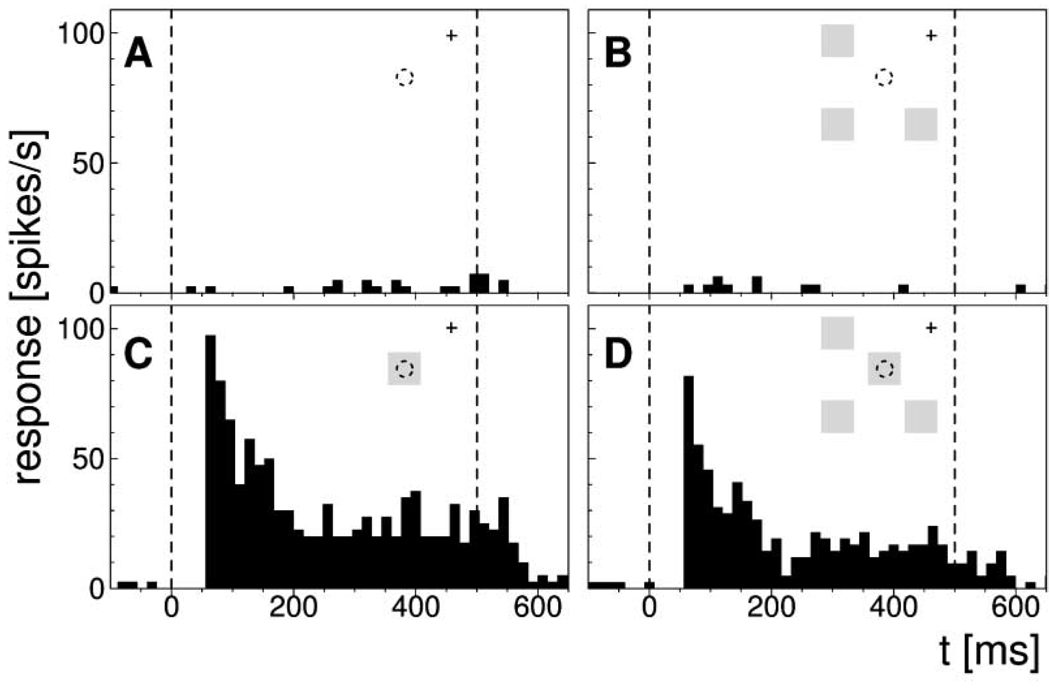
Figure 6. Responses Are Influenced by Remote Color Fields.
Response histograms for a neuron tested with stimuli and three remote color fields of the same chromaticity. Remote fields were 2° squares at a lateral distance of 5° from the receptive field, presented during the 500 ms stimulus period.
(A and B) Control conditions without stimulus.
(C and D) Responses to stimulus.
(D) When remote fields are presented in addition to the stimulus, the response is slightly reduced. Insets show stimulus configurations (circle, receptive field; cross, fixation spot; display elements are not drawn to scale).
Figure 7. Influence of Remote Color Fields.
Distributions of response ratios for stimuli with and without remote fields.
(A) Remote fields had same chromaticity as stimuli. Median: 0.88. Black data points with crosses indicate corresponding values for psychophysical data of eight subjects (median: 0.92).
(B) Remote fields and stimulus had opponent chromaticities. Median: 1.02. Note that in only a subset of experiments two remote field colors were used. The different results for the two conditions indicates that the influence of remote fields is color specific.
Perceptual Effects
The appearance of a color stimulus on a colored background can vary depending on the color of the background. Induction effects typically shift the perceived stimulus color away from the background color. Our data show that background color influences responses to chromatic stimuli in primary visual cortex in a qualitatively similar way. If these effects in V1 responses are relevant for color perception, we could expect a quantitative correspondence to the perceptual effects. We therefore compared the magnitudes of the chromatic interactions in the neural responses to induction effects in perception. We used data acquired in psychophysical experiments investigating lateral interactions in color perception (Wachtler et al., 2001a). In these experiments, observers judged the color of a 2° test field presented on either a neutral gray background or a colored background, with or without 2° remote fields 4.5° from the test field. Chromaticities and presentation times were the same as in the current study. The resulting data were perceptual color shifts, measured in cone contrast, induced by changes in the chromaticities of background or remote color fields.
To make the comparison between neural responses and perceptual effects, we assumed that our measured tuning curves, though individually tuned to different colors, were representative of the population of neurons encoding a single stimulus color after compensation for differences in preferred chromatic directions. We then assumed that perception of a certain color depends mainly on such a population, that is, on the neurons with the strongest responses. Under the further assumption of a linear relation between perceptual color shifts and underlying neural responses, we used the psychophysically measured values to calculate background modulation index ρbg and remote fields response ratio ρrem, respectively. The resulting data for eight human subjects are superimposed on the respective distributions in Figures 5A and 7A. For both indices, the values derived from human psychophysical data cluster around the centers of the distributions for the neural responses. This indicates that the magnitudes of the influences of both background color and remote colors are comparable in responses of V1 neurons and in perception.
For our comparison, we used data from experiments where the distance of the remote fields to the stimulus was similar (4.5° in human psychophysics, 4° to 6° in the physiology experiments). While we found the influences of the remote fields to range over at least 10° in perception (Wachtler et al. 2001a), occasional tests of neural responses with remote fields at 8° did not yield measurable effects of the remote fields. We did not investigate the spatial extent systematically, but these observations seem to indicate that the spatial range of chromatic interactions is more limited in primary visual cortex than in perception.
Estimating Stimulus Color from Population Responses
For methodological reasons, our quantitative comparison of neural and psychophysical data was restricted to the conditions where background and stimulus were along the same chromatic direction. At a qualitative level, we can investigate further induction effects. The alterations observed in tuning curves when stimuli were presented on chromatic background indicate that the same chromaticity can evoke different responses in V1 neurons. Conversely, a certain response pattern of color-selective neurons in V1 will not correspond to a unique stimulus chromaticity. Similar effects are known in perception and are often associated with strategies for achieving color constancy. Under different illumination, light from the same object will have different spectral composition, but nevertheless the color of the object typically looks similar. This is illustrated in Figure 8A, where two schematic scenes with different backgrounds are depicted, demonstrating interactions between stimulus and background chromaticities. We asked whether V1 neurons may implement some of these chromatic interactions.
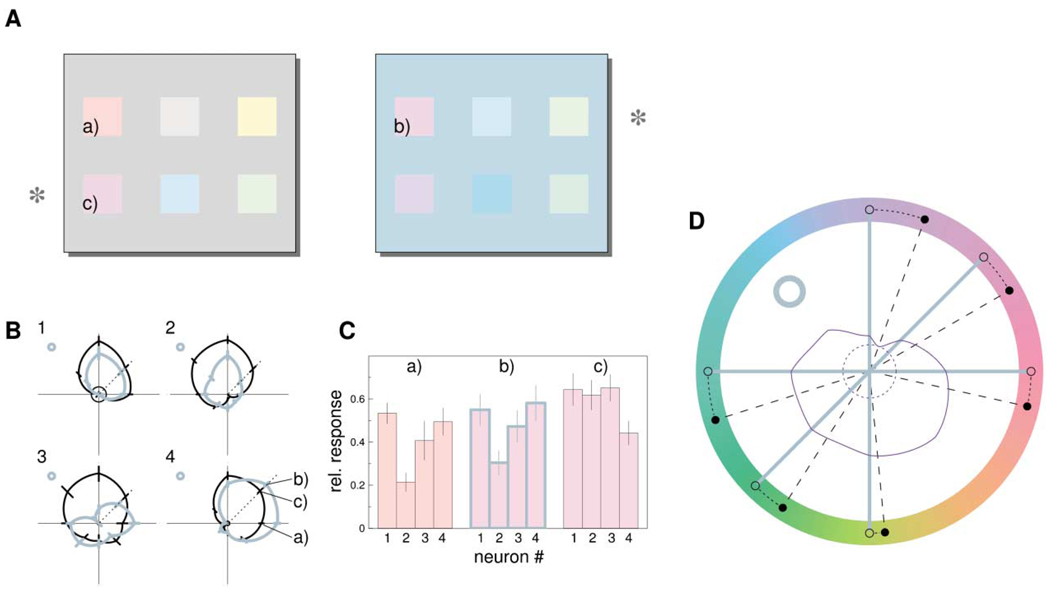
Figure 8. Estimation of Stimulus Color from Population Response.
(A) Perceptual effect of background color. The rows of color patches marked by asterisks are physically identical, but they are displayed on different backgrounds and therefore look different. For example, patch (b) looks more similar to the physically different patch (a) than to the physically identical patch (c).
(B) Four of the tuning curves of Figure 3, rotated in color space such that the respective directions of the background chromaticities (gray circles) are aligned, to mimic the background conditions in (A). Responses to patches (a)–(c) were estimated from the data points on the appropriate tuning curves in the 0° and 45° directions.
(C) Estimated responses to patches (a)–(c) for the four neurons in (B). Responses are scaled relative to maximum firing rate for each neuron individually. The response pattern elicited by patch (b) is more similar to that for patch (a) than to that for (c), corresponding to the perceptual situation.
(D) Induced color shifts estimated from 94 tuning curve pairs. For responses to six stimulus chromaticities (open dots) on bluish background (bluish circle), those chromaticities (black dots) were determined that yielded the most similar responses when presented on a gray background. Responses were considered as 94-dimensional vectors and similarity was measured by Euclidean distance. The dark purple curve shows, for the 90° (+S) stimulus on bluish background, the response vector distance as function of chromatic direction. The minimum occurs at a direction that is shifted from the 90° direction, away from the background direction. The dashed dark purple circle denotes the minimum distance, for easier visual inspection of the distance curve. For the other directions tested, similar shifts were obtained. Thus, qualitatively, the background has the same inducing effect as in perception.
To illustrate our approach, we first consider the responses of four neurons (Figure 8B) to color patches on different backgrounds. The chromatic background used to determine tuning curves was typically different for different neurons. To mimic identical background conditions, we rotated the tuning curves in the (L-M,S) plane such that all background chromaticities fell on the same direction. Thus, we assume that tuning properties and background effects in the neural population do not depend on chromatic direction. The color patches (a) and (c) in Figure 8A have chromaticities that roughly correspond to 0° (+L-M) and 45° directions in the (L-M,S) plane, respectively. The responses they elicit can be estimated by the corresponding data points on the tuning curve for neutral background. Color patch (b) has the same chromaticity as (c) but is displayed on a bluish background. Therefore, the response it elicits corresponds to the 45° data point on the tuning curves for chromatic backgrounds. The respective responses for the four neurons are plotted in Figure 8C. The response patterns for (a) and (b) are highly similar and are different from the pattern for (c), although patches (b) and (c) are physically identical. This corresponds to the perceptual similarity of patches (a) and (b).
Applying this approach to our population of 94 neurons, we determined for six of our stimuli (open black dots in Figure 8D) presented on a bluish background, the respective chromaticities on a neutral background that would elicit the most similar responses. Since the tuning curves were obtained for fixed stimulus contrast, we excluded the stimuli in the direction of the background chromaticity and the opposite direction. They would be expected to lead mainly to contrast changes rather than hue changes, the case which has been considered above (Figure 5). Responses as a function of chromatic direction were estimated from the individual tuning curves obtained by second-order polynomial interpolation of the data, and population responses were represented as 94-dimensional vectors. For each of the six stimuli, we then determined the chromatic direction for which the response vector for the neutral background condition had minimal distance to the response vector for that stimulus on the chromatic background. Vector difference was measured by Euclidean distance, but other measures gave similar results. The obtained chromaticities were shifted from the original stimulus chromaticities in the direction away from the background color, toward the opponent color. This is in close correspondence to induction effects in color perception, where, for example, stimuli on bluish background appear less blue, or more yellow, than on gray background (Figure 8A).
Discussion
Our results regarding chromatic tuning of V1 neurons in awake monkeys are largely consistent with reports of previous studies using anaesthetized animals. Chromatic selectivity is diverse, and most cells showed responses to isoluminant as well as to nonisoluminant stimuli, in agreement with earlier studies (Lennie et al., 1990; Johnson et al., 2001).
We did not attempt to find for each cell the stimulus parameters that would evoke the strongest responses, as was done in most previous studies. Apart from slight size changes, the stimuli were the same for all neurons and were identical to the stimuli used in psychophysical experiments. This enabled us to analyze the data as responses of a population of neurons and to compare them to perceptual experiments done with the same stimuli.
Owing to the properties of our stimuli, we preferentially recorded from neurons that responded strongly to homogeneous color patches, and we may have excluded cells that respond to spatial chromatic contrast (Michael, 1978b; Lennie et al., 1990; Johnson et al., 2001; Conway, 2001). It is not clear whether cells that respond preferentially to chromatic edges and cells that prefer homogeneous stimuli form distinct classes, or whether this just reflects different spatial frequency tuning (Johnson et al., 2001). Spatial opponency, often considered an important property of color-selective neurons, was largely ignored by our choice of stimuli. They were designed to ensure that the classical receptive field was covered. Nevertheless, relatively strong lateral interactions were found. In our study, we were primarily interested in the representation of chromatic stimuli under conditions comparable to typical situations in which we have to judge the color of an object. Spatial chromatic contrast probably plays a role in the detection of object borders and the visual segmentation of scenes (Hurlbert and Poggio, 1988), but experiments on color appearance usually employ extended stimuli with dimensions on the order of degrees of visual angle. We argue that, likewise, we typically judge the colors of natural objects from more or less extended surface regions. Our stimuli are in accordance with these spatial conditions, as well as with the contrast range (Tadmor and Tolhurst, 2000) and temporal constraints (Yarbus, 1967) in natural viewing. Therefore, our data are suitable for the comparison with properties of color perception.
Chromatic Tuning
Studies of color processing in the visual system often emphasize the relevance of color-opponent cells. Our results show that many cells that are not opponent nevertheless respond to color stimuli and exhibit chromatic tuning (Poggio et al., 1975; Gouras and Krüger, 1979), and therefore may contribute to color perception.
For isoluminant stimuli, we found that the directions of tuning peaks were continuously distributed. While this is in agreement with most previous studies (Thorell et al., 1984; Lennie et al., 1990; De Valois et al., 2000; Hanazawa et al., 2000), contrary observations have been reported (Ts’o and Gilbert, 1988).
The tuning properties of V1 cells indicate that the representation of chromatic information in the visual cortex is organized differently from that in the LGN. LGN neurons encode the chromatic component of a stimulus corresponding to one of the axes of cone-opponent color space, and thus the responses can be thought of as coordinate values in color space. It has been proposed that the transformation occurring in the primary visual cortex corresponds to a rotation of the chromatic axes toward the unique hues (De Valois et al., 2000). While our results are generally consistent with such an interpretation, they further indicate that the recoding between LGN and V1 is rather complex. First, the frequent occurrence of tuning widths inconsistent with cosine-tuning (see also De Valois et al., 2000) and the anisotropy of tuning functions in three-dimensional color space (Figure 1) show that chromatic tuning is not just a result of a linear combination of cone signals (cf. Hanazawa et al., 2000). Second, the distribution of tuning peak directions suggests that there are not only clusters in the regions corresponding to red, yellow, green, and blue (Vautin and Dow, 1985; De Valois et al., 2000), but also, at least as pronounced, for purple. Such cells with preference for extraspectral colors have been described before for both striate (Dow and Vautin, 1987) and extrastriate cortex (Zeki, 1980). Third, in contrast to encoding in the LGN, the continuum of tuning directions indicates a distributed code for chromatic stimuli, where each chromaticity is represented by a population of neurons with different peak sensitivities but overlapping tuning curves.
The clear shift of the main chromatic directions, together with the strong representation of the red-orange-yellow region, coincides with properties of efficient codes for chromatic stimuli revealed by analysis of natural scenes (Wachtler et al., 2001b). Efficient representations for natural chromatic stimuli show chromatic tuning that deviates systematically from the cone-opponent color space axes, with a preference for an opponency axis that matches the chromatic direction of the main peak in the distribution of V1 tuning curves (Figure 2). This suggests that, while the coding scheme in the LGN may be constrained by the requirement to transfer the information through a limited number of optic nerve fibers (Lee et al., 2002), responses in visual cortex may achieve a more efficient representation for natural chromatic stimuli.
Contextual Effects
To test for lateral chromatic interactions, we presented the stimuli on backgrounds of different chromaticities. In most cases, chromatic tuning was different for different background chromaticities, suggesting that responses of V1 cells do not represent merely the chromaticity of a stimulus at the receptive field location, but to some degree also the chromatic contrast of the stimulus to the background. The average strength of this effect is comparable to color induction effects in human perception, as measured under similar stimulus conditions. The distribution of the magnitude of this effect for single neurons is broader than the interindividual variability in the perceptual data, but this would be expected if stimulus color is represented by a population of many neurons. Likewise, the effect of remotely presented color patches, for considerable distances up to 6°, is comparable in strength to the perceptual effect. The main difference between the neural response recorded in macaque V1 and human psychophysics that we observed was with the spatial extent of the color contrast and surround effects, which may reflect the smaller sizes of V1 receptive fields and indicate the need to integrate their responses at higher levels of the visual system. This conclusion is currently based on limited observations. Further studies are needed to systematically investigate the spatial extent of chromatic interactions.
Color-selective neurons with purely suppressive (“silent”) surrounds have been described by several studies (Michael, 1978a; Livingstone and Hubel, 1984; Ts’o and Gilbert, 1988). Our results, however, show clearly not only suppression but also response enhancement, depending on the chromatic relation between stimulus and background. Thus, the influence of the background in our experiments is a color-specific modulation of the response.
Contextual influences in V1 have been reported to occur for color stimuli when color was a cue for image segmentation (Zipser et al., 1996). While our results would be consistent with an interpretation of the coding of figure-ground relations or stimulus saliency (Nothdurft et al., 1999), the correspondence with color induction effects suggests that these interactions play a role in color appearance. In previous experiments, lateral interactions in responses to color stimuli were studied in V1 and V4 and found to be compatible with perception only in V4 (Zeki, 1983a, 1983b). Large stimuli were used in these experiments and the more limited spatial extent of interactions in V1 may account for this conclusion (Maunsell and Newsome, 1987). Our findings support this interpretation, but other methodological differences, such as the use of anaesthetized versus awake animals, or selection of a different set of neurons, may have also contributed to the differences in results.
Our comparison between neural responses and perception is somewhat indirect, comparing neural activity in the monkey with human percepts. To confirm our results, experiments would be necessary where monkeys are indicating their color percepts during recording of the neural responses. However, experimental evidence so far strongly supports the notion that color perception in monkeys and in humans is highly similar (De Valois et al., 1974; Loop and Crossman, 2000). Thus, it is unlikely that such a control experiment would lead to a different result.
Conclusions
In summary, our results indicate that the representation of chromatic stimuli in primary visual cortex is qualitatively different from that in the LGN. Moreover, we found that this representation shares important aspects with the color percept, in particular with respect to color induction. Together with previous results on color processing in the visual system, our findings suggest that the visual processing leading to the color percept is distributed over a series of processing stages (cf. De Valois and De Valois, 1993). Chromatic interactions that may be relevant for perception have been found to occur at many levels, from retina (Pöppel, 1986) and LGN (Kastner et al., 1992) to V4 (Zeki, 1983b) and beyond (Komatsu et al., 1992). Likewise, primary visual cortex certainly contributes in this hierarchy and, as for many other visual aspects, plays an important role in the neural processing that leads from the sensory signals to our percept.
Experimental Procedures
Animal Preparation and Training
Two adult female rhesus monkeys (Macaca mulatta) were used in this study. Experimental protocols were approved by the Salk Institute Animal Care and Use Committee and conform to United States Department of Agriculture regulations, the United States Public Health Service Policy on humane care and use of laboratory animals, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Monkeys were prepared for the experiments using conventional techniques, which have been described in detail elsewhere (e.g., Dobkins and Albright, 1994; Chaudhuri and Albright, 1997). Surgical procedures were performed under aseptic conditions using barbiturate or halothane anesthesia. Animals were given prophylacitc antibiotics (30 mg/kg Keflin at 2 hr intervals during surgery) and postsurgical analgesics (0.03 mg/kg buprenophorine i.m. in 12 hr intervals for 3 days). Prior to behavioral training, a search coil for measuring eye position was implanted in one eye (Robinson, 1963; Judge et al., 1980), and a stainless steel post for head restraint was fastened to the skull with stainless steel screws. After fixation training, a stainless steel recording chamber (Crist Instruments, Damascus, MD) was affixed to the skull over the location of V1. Positioning of the recording chamber was guided by cranial magnetic resonance imaging (MRI) scans obtained before surgery.
During the experiments, monkeys were seated in a primate chair (Crist Instruments) in a quiet room. The head post was bolted to the frame of the primate chair to maintain head position. Animals were required to fixate a small fixation target for the duration of each trial (2500 ms). After each successful trial, animals were given small (0.1–0.15 ml) juice rewards.
Electrophysiological Recordings
Extracellular potentials from single isolated neurons were recorded with paralyene-coated tungsten microelectrodes (Frederick Haer, Brunswick, ME). Electrodes were lowered into the brain through a stainless steel guide tube by way of a hydraulic microdrive. Electrode, guide tube, and microdrive assembly were attached to the recording chamber by way of an x-y stage (Crist Instruments, Damascus, MD). Amplified electrical activity from the cortex was passed to a data acquisition system for spike detection and sorting (Alpha Omega Engineering, Nazareth, Israel).
Once a neuron was isolated, its receptive field was mapped using flashed and moving bars of different sizes (0.1–1.0), orientations, and colors. The stimulus parameters were under the experimenter’s control during this procedure. The area in which a response could be elicited by the stimuli was determined to define the boundaries of the receptive field of the neuron. In addition, the degree of orientation tuning and preferred orientation were estimated. The neurons recorded had receptive field locations at eccentricities between 2° and 5°.
Visual Stimulation
Stimulus displays were generated by an 8-bit graphics board (Number Nine Computer Corporation, Cambridge, MA; Pepper SGT, 640 × 480 pixels, 60 Hz) and presented on a calibrated CRT screen (SONY GDM-2000TC). Stimulus presentation, as well as data acquisition and behavioral control, were controlled using the CORTEX 5.7 software (Laboratory of Neuropsychology, National Institute of Mental Health). A spectroradiometer (PR-650; PhotoResearch, Chatsworth, CA) was used to calibrate the display and verify the chromaticities in the displays.
Colors were specified in a color space similar to the one used by Derrington et al. (1984), which is based on opponent representation of cone responses (Luther, 1927; MacLeod and Boynton, 1979) but specifies chromaticities in terms of cone contrasts with respect to a reference color. Cone excitations were calculated on the basis of the human cone fundamentals proposed by Stockman et al. (1993). The origin of the color space corresponded to the chromaticity of the gray background to which the subject adapted. The three coordinate axes of our color space corresponded to L- versus M-cone (“L-M” axis), S-cone versus L- and M-cone (“S” axis), and achromatic luminance variation (“Lum” axis), measured in cone contrast with respect to the neutral gray background. L and M variations along the L-M axis were antagonistic such that the sum of L and M cone excitations was constant, and contrast was measured as the difference of L and M cone contrasts, i.e., positive values corresponded to increasing L- and decreasing M-cone excitation. Positive values on the y axis corresponded to positive S-cone contrast (MacLeod and Boynton, 1979).
Stimuli
Stimuli were homogeneous color squares with sizes between 2.5° and 4.5°, centered on and at least twice as large as the receptive field of the neuron under investigation, and were flashed for 500 ms. Stimulus colors were chosen from different directions in color space. Eight colors were chosen in the (L-M,S) plane, their chromatic directions corresponding to polar angles in steps of 45°, thus including pure S-cone stimuli. In addition, pure L-cone, pure M-cone, and achromatic stimuli were used. All stimuli had the same fixed cone contrast (cs = 0.15) to the gray background. In each trial, the stimulus was chosen randomly from the set of stimuli used in the experiment.
In some experiments, the following additional stimuli were used. Due to limited recording time, not all of these stimuli were used in each experiment. Sixteen stimuli with either higher or lower luminance than the eight stimuli in the (L-M,S) plane, but with the same cone contrast, were used to determine response contours in three-dimensional color space. Stimuli in the direction of the chromatic background (see below), with cone contrasts between 0.05 and 0.2 in steps of 0.05, were used to determine response magnitude as a function of stimulus contrast.
Background
Animals were adapted to a neutral gray background (CIE (x,y) = (0.314, 0.325), 41.2 cd/m2 ), on which the stimuli were presented in the standard condition. For each experiment, a second background color was chosen. This background was isoluminant with the standard gray background. Its color was in the direction of one of the eight stimuli in the (L-M,S) plane, typically a direction for which the neuron under investigation showed a clear response. It was of lower contrast (cb = 0.10) with respect to the neutral background than the stimuli. For each trial, it was chosen randomly whether the neutral gray background remained throughout the trial, or whether the background changed to the alternate color during stimulus presentation. The chromatic background was displayed only during the 500 ms when the stimulus was present.
Remote Fields
Remote fields were three 2° squares with the same cone contrast (cr = 0.15) as the stimuli, presented during the 500 ms stimulus presentation period at a distance of 4° to 6° from the receptive field. They were placed at three of the four corners of a hypothetical square centered on the receptive field. No remote patch was displayed at the corner that was closest to the fixation point. In experiments with remote fields, only one stimulus color was used, and remote fields were either of the same color or the opponent color. From trial to trial, it was decided randomly whether remote fields were presented or not, and, if applicable, what color they had.
Experimental Procedure
To find neurons responsive to color stimuli, 4° color squares, isoluminant or achromatic, were flashed rapidly (100 ms flashes every 350 ms) in randomized order. Once a neuron responsive to any of these stimuli was isolated, its receptive field properties were determined as described above. The stimulus size was adjusted to a length of two to four times of the longest elongation of the receptive field.
To determine chromatic tuning, the color stimuli were selected in random order and flashed for 500 ms. Neural responses were recorded for 1500 ms around this period. Response strength was measured as mean spike rate in a time window 50–150 ms after stimulus onset. In addition to the neutral gray background, one of the eight possible alternate background chromaticities was selected. This was typically a chromaticity to which the neuron showed a clear response, in order to use colors that were relevant for the neuron’s response. In subsequent trials, either this chromaticity or the neutral gray was chosen randomly as background color during the 500 ms stimulus presentation period.
Acknowledgments
We thank J. Costanza and K. Sevenbergen for excellent technical support, Greg Horwitz, Anya Hurlbert, and Christian Wehrhahn for valuable discussions and comments on the manuscript, and the anonymous reviewers for helpful suggestions. This work was supported by the Alfred P. Sloan Foundation and the National Eye Institute (TDA). T.D.A. and T.J.S. are Investigators of the Howard Hughes Medical Institute.
References
- Brown RO, MacLeod DIA. Color appearance depends on the variance of surround colors. Curr. Biol. 1997;7:844–849. doi: 10.1016/s0960-9822(06)00372-1. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Albright TD. Neuronal responses to edges defined by luminance vs. temporal texture in macaque area V1. Vis. Neurosci. 1997;14:949–962. doi: 10.1017/s0952523800011664. [DOI] [PubMed] [Google Scholar]
- Conway BR. Spatial structure of cone inputs to color cells in alert macaque primary visual cortex (V-1) J. Neurosci. 2001;21:2768–2783. doi: 10.1523/JNEUROSCI.21-08-02768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annu. Rev. Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. A multi-stage color model. Vision Res. 1993;33:1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Abramov I, Jacobs GH. Analysis of response patterns of LGN cells. J. Opt. Soc. Am. 1966;56:966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Morgan HC, Polson MC, Mead WR, Hull EM. Psychophysical studies of monkey vision I. Macaque luminosity and color vision tests. Vision Res. 1974;14:53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Cottaris NP, Elfar SD, Mahon LE, Wilson JA. Some transformations of color information from lateral geniculate nucleus to striate cortex. Proc. Natl. Acad. Sci. USA. 2000;97:4997–5002. doi: 10.1073/pnas.97.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J. Physiol. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkins KR, Albright TD. What happens if it changes color when it moves? The nature of chromatic input to macaque visual area MT. J. Neurosci. 1994;14:4854–4870. doi: 10.1523/JNEUROSCI.14-08-04854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow BM, Vautin RG. Horizontal segregation of color information in the middle layers of foveal striate cortex. J. Neurophysiol. 1987;57:712–739. doi: 10.1152/jn.1987.57.3.712. [DOI] [PubMed] [Google Scholar]
- Gouras P. Opponent-colour cells in different layers of foveal striate cortex. J. Physiol. 1974;238:583–602. doi: 10.1113/jphysiol.1974.sp010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Krüger J. Responses of cells in foveal visual cortex of the monkey to pure color contrast. J. Neurophysiol. 1979;42:850–860. doi: 10.1152/jn.1979.42.3.850. [DOI] [PubMed] [Google Scholar]
- Hanazawa A, Komatsu H, Murakami I. Neural selectivity for hue and saturation of colour in the primary visual cortex of the monkey. Eur. J. Neurosci. 2000;12:1753–1763. doi: 10.1046/j.1460-9568.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- Hurlbert A, Poggio T. A network for image segmentation using color. Adv. Neural Inf. Process. Syst. 1988;1:297–304. [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nat. Neurosci. 2001;4:409–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Crook JM, Pei X, Creutzfeldt OD. Neurophysiological correlates of colour induction on white surfaces. Eur. J. Neurosci. 1992;4:1079–1086. doi: 10.1111/j.1460-9568.1992.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Komatsu H. Neural representation of the luminance and brightness of a uniform surface in the macaque primary visual cortex. J. Neurophysiol. 2001;86:2559–2570. doi: 10.1152/jn.2001.86.5.2559. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Van Essen DC. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J. Neurophysiol. 1992;67:961–980. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Ideura Y, Kaji S, Yamane S. Color selectivity of neurons in the inferior temporal cortex of the awake macaque monkey. J. Neurosci. 1992;12:408–424. doi: 10.1523/JNEUROSCI.12-02-00408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft JM, Brainard DH. Mechanisms of color constancy under nearly natural viewing. Proc. Natl. Acad. Sci. USA. 1999;96:307–312. doi: 10.1073/pnas.96.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land EH. Color vision and the natural image. part I. Proc. Natl. Acad. Sci. USA. 1959;45:115–129. doi: 10.1073/pnas.45.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-W, Wachtler T, Sejnowski TJ. Color opponency is an efficient representation of spectral properties in natural scenes. Vision Res. 2002;42:2095–2103. doi: 10.1016/s0042-6989(02)00122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J. Neurosci. 1990;10:649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Thier P, Wehrhahn C. Contextual influence on orientation discrimination of humans and responses of neurons in V1 of alert monkeys. J. Neurophysiol. 2000;83:941–954. doi: 10.1152/jn.2000.83.2.941. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loop MS, Crossman DK. High color-vision sensitivity in macaque and humans. Vis. Neurosci. 2000;17:119–125. doi: 10.1017/s0952523800171123. [DOI] [PubMed] [Google Scholar]
- Luther R. Aus dem Gebiet der Farbreizmetrik. Zeitschrift für technische Physik. 1927;8:540–558. [Google Scholar]
- MacLeod DIA, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J. Opt. Soc. Am. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- Martin PR. Colour processing in the primate retina: recent progress. J. Physiol. 1998;513:631–638. doi: 10.1111/j.1469-7793.1998.631ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JHR, Newsome WT. Visual processing in monkey extrastriate cortex. Annu. Rev. Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- Michael CR. Color vision mechanisms in monkey striate cortex: dual-opponent cells with concentric receptive fields. J. Neurophysiol. 1978a;41:572–588. doi: 10.1152/jn.1978.41.3.572. [DOI] [PubMed] [Google Scholar]
- Michael CR. Color vision mechanisms in monkey striate cortex: simple cells with dual opponent-color receptive fields. J. Neurophysiol. 1978b;41:1233–1249. doi: 10.1152/jn.1978.41.5.1233. [DOI] [PubMed] [Google Scholar]
- Motokawa K, Taira N, Okuda J. Spectral responses of single units in the primate visual cortex. Tohoku J. Exp. Med. 1962;78:320–337. doi: 10.1620/tjem.78.320. [DOI] [PubMed] [Google Scholar]
- Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC, Gallant JL, Van Essen DC. Response modulation by texture surround in primate area V1: correlates of “popout” under anesthesia. Vis. Neurosci. 1999;16:15–34. doi: 10.1017/s0952523899156189. [DOI] [PubMed] [Google Scholar]
- Poggio GF, Baker FH, Mansfield RJW, Sillito A, Grigg P. Spatial and chromatic properties of neurons subserving foveal and parafoveal vision in rhesus monkey. Brain Res. 1975;100:25–59. doi: 10.1016/0006-8993(75)90240-1. [DOI] [PubMed] [Google Scholar]
- Pöppel E. Long-range colour-generating interactions across the retina. Nature. 1986;320:523–525. doi: 10.1038/320523a0. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans. Biomed. Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Paradiso MA. Neural correlates of perceived brightness in the retina, lateral geniculate nucleus, and striate cortex. J. Neurosci. 1999;19:6145–6156. doi: 10.1523/JNEUROSCI.19-14-06145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein SJ, Desimone R. Spectral properties of V4 neurons in the macaque. J. Neurosci. 1990;10:3369–3389. doi: 10.1523/JNEUROSCI.10-10-03369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Context-dependent interactions and visual processing in V1. J. Physiol. (Paris) 1996;90:205–209. doi: 10.1016/s0928-4257(97)81424-6. [DOI] [PubMed] [Google Scholar]
- Stockman A, MacLeod DIA, Johnson NE. Spectral sensitivities of the human cones. J. Opt. Soc. Am. A. 1993;10:2491–2521. doi: 10.1364/josaa.10.002491. [DOI] [PubMed] [Google Scholar]
- Tadmor Y, Tolhurst DJ. Calculating the contrasts that retinal ganglion cells and LGN neurones encounter in natural scenes. Vision Res. 2000;40:3145–3157. doi: 10.1016/s0042-6989(00)00166-8. [DOI] [PubMed] [Google Scholar]
- Thorell LG, De Valois RL, Albrecht DG. Spatial mapping of monkey V1 cells with pure color and luminance stimuli. Vision Res. 1984;24:751–769. doi: 10.1016/0042-6989(84)90216-5. [DOI] [PubMed] [Google Scholar]
- Ts’o DY, Gilbert CD. The organization of chromatic and spatial interactions in the primate striate cortex. J. Neurosci. 1988;8:1712–1727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valberg A. Unique hues: an old problem for a new generation. Vision Res. 2001;41:1645–1657. doi: 10.1016/s0042-6989(01)00041-4. [DOI] [PubMed] [Google Scholar]
- Vautin RG, Dow BM. Color cell groups in foveal striate cortex of the behaving macaque. J. Neurophysiol. 1985;54:273–292. doi: 10.1152/jn.1985.54.2.273. [DOI] [PubMed] [Google Scholar]
- Wachtler T, Albright TD, Sejnowski TJ. Nonlocal interactions in color perception: nonlinear processing of chromatic signals from remote inducers. Vision Res. 2001a;41:1535–1546. doi: 10.1016/s0042-6989(01)00017-7. [DOI] [PubMed] [Google Scholar]
- Wachtler T, Lee T-W, Sejnowski TJ. Chromatic structure of natural scenes. J. Opt. Soc. Am. A. 2001b;18:65–77. doi: 10.1364/josaa.18.000065. [DOI] [PubMed] [Google Scholar]
- Wesner MF, Shevell SK. Color perception within a chromatic context: changes in red/green equilibria caused by noncontiguous light. Vision Res. 1992;32:1623–1634. doi: 10.1016/0042-6989(92)90155-c. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J. Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye Movements and Vision. New York: Plenum; 1967. [Google Scholar]
- Zeki S. The representation of colours in the cerebral cortex. Nature. 1980;284:412–418. doi: 10.1038/284412a0. [DOI] [PubMed] [Google Scholar]
- Zeki S. Colour coding in the cerebral cortex: the reaction of cells in monkey visual cortex to wavelengths and colours. Neuroscience. 1983a;9:741–765. doi: 10.1016/0306-4522(83)90265-8. [DOI] [PubMed] [Google Scholar]
- Zeki S. Colour coding in the cerebral cortex: the responses of wavelength-selective and colour-coded cells in monkey visual cortex to changes in wavelength composition. Neuroscience. 1983b;9:767–781. doi: 10.1016/0306-4522(83)90266-x. [DOI] [PubMed] [Google Scholar]
- Zipser K, Lamme VAF, Schiller PH. Contextual modulation in primary visual cortex. J. Neurosci. 1996;16:7376–7389. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]