Scheme 3. Synthesis of (+)-ipalbidinea.
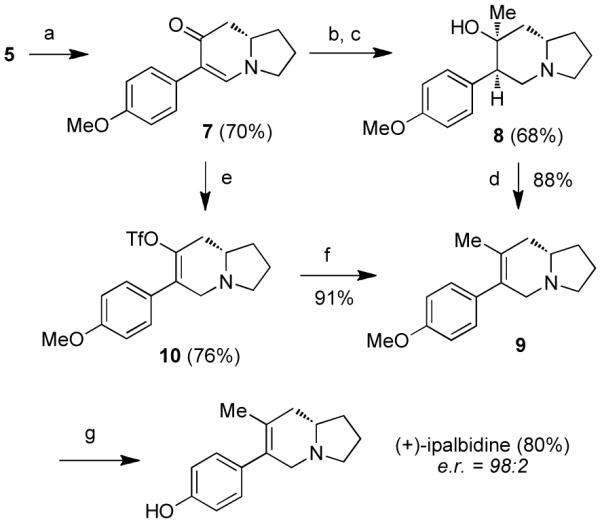
aReagents and conditions: (a) Pd(OAc)2 (30 mol%), Cu(OAc)2 (3 equiv), PMP–BF3K, K2CO3, t-BuOH/AcOH/DMSO (20:5:1), 60 °C; (b) L-Selectride, THF, −78 to 0 °C; (c) MeLi, THF, −78 °C; (d) SOCl2, pyridine, THF, −30 to −10 °C; (e) L-Selectride, THF, −78 to 0 °C; then Comins’ reagent, −78 to 0 °C; (f) Pd(PPh3)4 (10 mol%), MeZnBr, THF, 60 °C; (g) BBr3, CH2Cl2, −78 °C to rt. PMP = p-methoxyphenyl.
