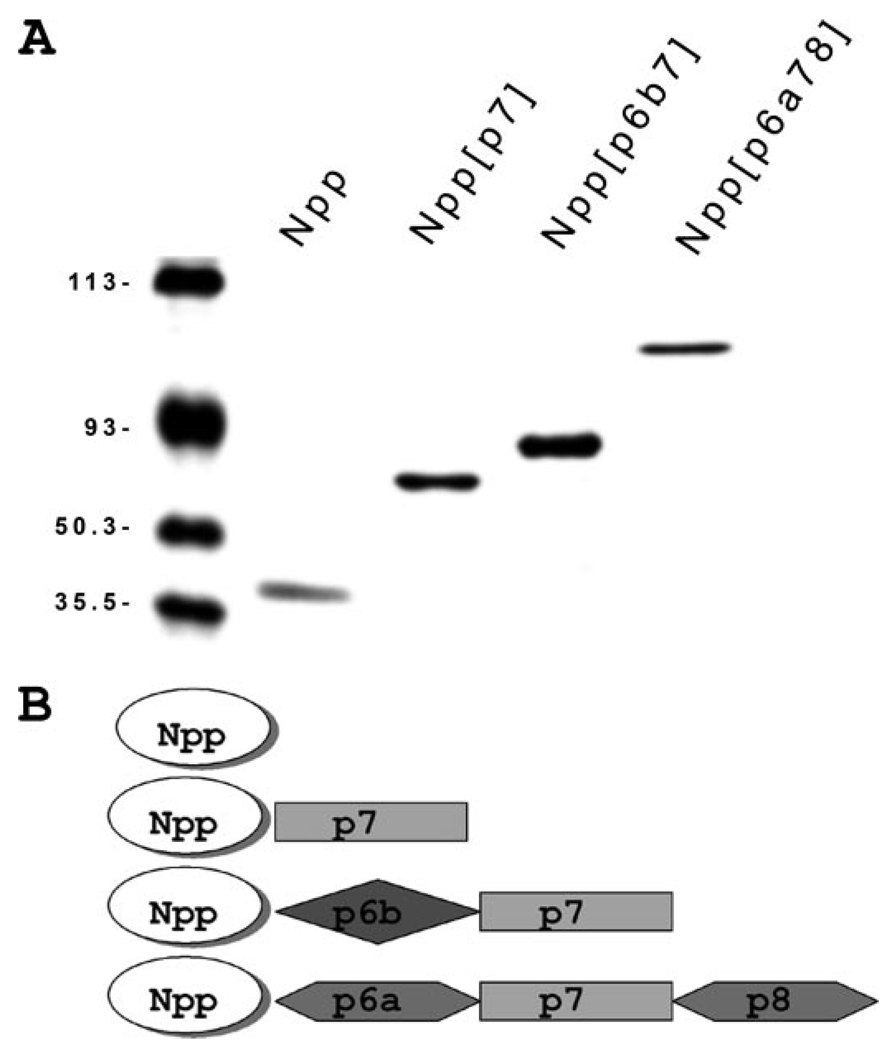
FIGURE 2. Protein expression and purification of recombinant fragments of collagen α1(XI) chain Npp and the three most prevalent amino-terminal domain splice variants.
A, purified recombinant collagen α1(XI) fragments were analyzed by SDS-polyacrylamide gel electrophoresis using a 10.5% acrylamide gel. Protein fragments generated for this study include: the amino propeptide (Npp), a fragment containing p7 of the variable region adjacent to the Npp domain (Npp[p7]), a fragment representing the amino-terminal domain with p6b and p7 adjacent to the Npp domain (Npp[p6b7]), and a fragment containing p6a, p7, and p8 adjacent to the Npp domain (Npp[p6a78]). Note that p6b7 is predicted to contain a heparan sulfate binding site. Note also that p6a78 is the largest, most negatively charged of the splice variants of α1(XI). Molecular weight markers are included in the left-hand lane. B, modular representation of the recombinant fragments used in this study, corresponding to proteins shown in A.