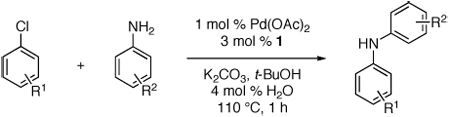
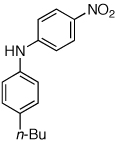
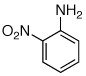
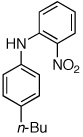
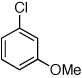
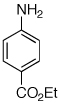
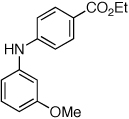
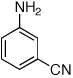
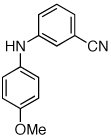
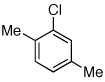
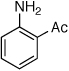
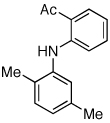
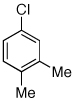
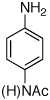
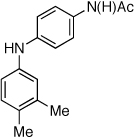
Table 2.
Coupling of Electron-Deficient Anilines
Reaction conditions: 1 min preactivation at 110 °C with 4 mol % H20, 1 mol % Pd(OAc)2, and 3 mol % 1 in 2 mL/mmol of t-BuOH; 1.0 equiv aryl chloride, 1.2 equiv amine, and 1.4 equiv K2CO3.
Yields represent isolated yields (average of 2 runs).
Reaction time of 2 h.
2.5 equiv of K2CO3 was used.