Abstract
Elongation of leaves and stem is a key trait for survival of terrestrial plants during shallow but prolonged floods that completely submerge the shoot. However, natural floods at different locations vary strongly in duration and depth, and, therefore, populations from these locations are subjected to different selection pressure, leading to intraspecific variation. Here, we identified the signal transduction component that causes response variation in shoot elongation among two accessions of the wetland plant Rumex palustris. These accessions differed 2-fold in petiole elongation rates upon submergence, with fast elongation found in a population from a river floodplain and slow elongation in plants from a lake bank. Fast petiole elongation under water consumes carbohydrates and depends on the (inter)action of the plant hormones ethylene, abscisic acid, and gibberellic acid. We found that carbohydrate levels and dynamics in shoots did not differ between the fast and slow elongating plants, but that the level of ethylene-regulated abscisic acid in petioles, and hence gibberellic acid responsiveness of these petioles explained the difference in shoot elongation upon submergence. Since this is the exact signal transduction level that also explains the variation in flooding-induced shoot elongation among plant species (namely, R. palustris and Rumex acetosa), we suggest that natural selection results in similar modification of regulatory pathways within and between species.
In plant communities characterized by hydrological gradients the distributions of individual plant species are distinct (Silvertown et al., 1999; Bartelheimer et al., 2010) and strongly affected by plant traits that confer flood tolerance and drought tolerance (van Eck et al., 2004; Lenssen and de Kroon, 2005). Environmental fluctuations such as flooding regimes act as a filter that prevents certain species from invading flood-prone environments due to the lack of specific suites of functional traits (Keddy, 1992).
During the course of evolution, many plant species have developed adaptive traits to survive flooding (Bailey-Serres and Voesenek, 2008; Colmer and Voesenek, 2009). One strategy that plants adopt to overcome the exhaustion of energy when submerged is to limit growth until flooding subsides. In certain rice (Oryza sativa) varieties underwater growth is inhibited by the SUB1A-1 gene, coding for a transcription factor that belongs to the APETALA2/ERF subfamily (Fukao et al., 2006; Xu et al., 2006). The limitation of elongation in submerged rice is achieved via a decreased responsiveness to GAs arising from elevated levels of DELLA proteins that repress GA-induced growth (Fukao and Bailey-Serres, 2008). Such a reduced elongation response is beneficial only if submergence is relatively short lasting and/or deep and when the plants’ potential for carbohydrate storage is large enough to fuel respiration under water.
However, when the environment is characterized by prolonged, but relatively shallow floods, escape from submergence seems to be a more beneficial strategy (Voesenek et al., 2004). To achieve this, some plant species elongate their stems or petioles to keep up with rising water levels or to grow above standing flood water (Ridge, 1987; Kende et al., 1998; Voesenek et al., 2006; Jackson, 2008). These shoot elongation responses are regulated by the interplay of several plant hormones. Gaseous ethylene is continuously produced and physically trapped within the plant during submergence (Ku et al., 1970; Musgrave et al., 1972). Accumulated ethylene reduces abscisic acid (ABA) levels by inhibiting ABA biosynthesis as suggested by the down-regulation of the 9-cis-epoxycarotenoid dioxygenase (NCED) ABA biosynthesis gene family (Benschop et al., 2005) and by increasing ABA degradation (Yang and Choi, 2006; Saika et al., 2007). This decline of ABA releases its repression of GA biosynthesis and thus facilitates the increase of the bioactive GA concentration in the submerged tissues (Benschop et al., 2006). In addition, sensitivity to GA is also enhanced by submergence and ethylene (Hoffmann-Benning and Kende, 1992; Rijnders et al., 1997), through yet unknown mechanisms. Recently, two SNORKEL genes (SK1 and SK2) were isolated in rice (Hattori et al., 2009). These genes belong to the same APETALA2/ERF subfamily as the SUB1A-1 gene and are involved in elongation of rice when submerged. The SK genes act upstream of GA, but it is not yet known whether they interfere with GA biosynthesis or action. Downstream targets for these hormonal growth control pathways include cell wall loosening proteins, such as expansins, which loosen the otherwise rigid cell wall (Choi et al., 2003; Cosgrove, 2005) to allow for turgor-driven cell elongation during submergence (Cho and Kende, 1997a, 1997b; Vreeburg et al., 2005), which drives shoot elongation (Métraux and Kende, 1984; Voesenek et al., 1990). Fast underwater elongation requires energy and carbon, and, therefore, depends on the availability of nonstructural carbohydrates (Groeneveld and Voesenek, 2003). It has been shown that submergence can induce mobilization of starch and translocation of newly fixed carbon to the elongating tissues (Raskin and Kende, 1984). Although it is clear that these various hormones, carbohydrates, and cell wall loosening proteins are needed for the elongation response, little is known about which parts of the signal transduction pathway cause differences among and within naturally occurring species. In contrast to wild species, more detailed information is available to explain variation in underwater elongation in cultivated rice varieties. In this species the APETALA2/ERF genes control whether underwater elongation is inhibited or stimulated (Voesenek and Bailey-Serres, 2009).
Rumex palustris is a wetland species, showing a clear shoot elongation response upon submergence (Voesenek et al., 1990). This elongation is crucial to escape the long shallow floods it experiences in many of its natural habitats (Voesenek et al., 2004) and emergence results in higher biomass compared to continuously submerged plants (Pierik et al., 2009). However, floods in natural environments differ widely in terms of duration and depth (Vervuren et al., 2003). Therefore, shoot elongation may have been under different selection pressures in different flooding regimes. Interestingly, plant life in aquatic environments evolved from terrestrial ancestors more than 200 times, independently (Jackson et al., 2009), suggesting that flood adaptive traits can evolve relatively easily as the result of a few mutations, possibly because the basic signal transduction mechanisms and the growth machinery required are present in most species.
Studying intraspecific variation for flood adaptive traits offers the opportunity to elucidate which regulatory steps in the signal transduction pathways show within-species variation and can thus potentially be subject to selection pressures and to microevolutionary changes. While microevolutionary studies on phenotypically plastic traits are common (Huber et al., 2004, 2009), the use of genetically diverging material that evolved in natural conditions to elucidate selection on regulatory components is only emerging (Sultan, 2007; Wilczek et al., 2009). Here we report on the critical differences in signal transduction components between two natural accessions of R. palustris, one with strong petiole elongation under water, and another with modest elongation. We tested the effects of the known regulatory components in relation to the differences in underwater elongation between these two accessions. Our results indicate that neither carbohydrates nor expansins explained the intraspecific variation. However, we found that the slow elongating accession maintained higher ABA levels during submergence and that due to these relatively high levels this accession has reduced sensitivity to GA.
RESULTS AND DISCUSSION
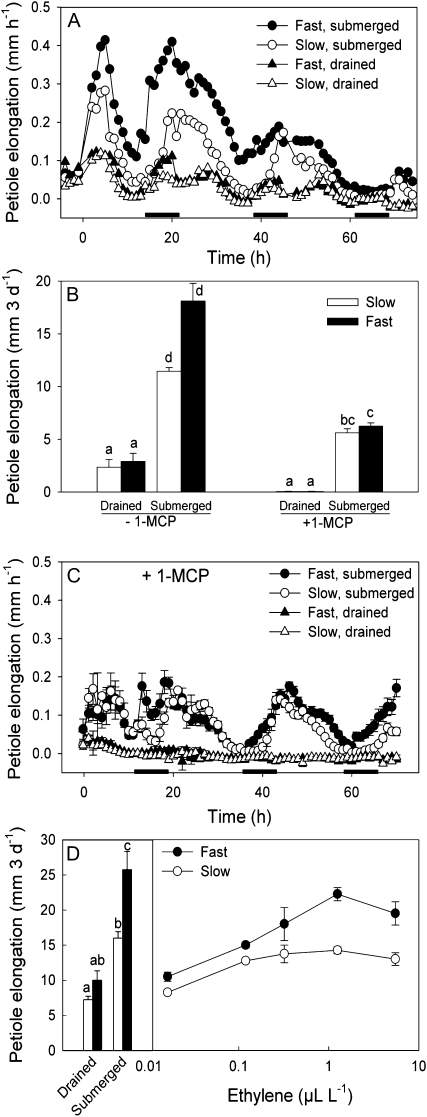
Flooding-induced shoot elongation enables submerged plants to restore contact with light and air, and thus improves aerial photosynthesis and aerobic respiration. This plant trait predominantly occurs in environments characterized by prolonged, but relatively shallow flooding events (Voesenek et al., 2004). Leaf emergence out of flood water resulting from fast shoot elongation benefits biomass accumulation if these plants contain sufficient aerenchyma to facilitate gas diffusion through the entire plant (Colmer, 2003; Pierik et al., 2009). The objective of this work was to elucidate which differences in regulatory steps in the signaling pathway can explain genetic variation in flooding-induced shoot elongation. This was studied in two natural accessions of R. palustris that are characterized by different petiole elongation rates when submerged. Although both accessions responded to flooding with enhanced elongation rates (Fig. 1A), it resulted on average in 50% higher petiole elongation rates and thus longer petioles in the accession originating from a river floodplain compared to the accession originating from a lakeside community (Fig. 1B). These differences were subsequently traced down to different steps in the regulatory pathways.
Figure 1.
Ethylene controls variation in submergence-induced elongation of the third-oldest petiole of the fast and slow accessions of R. palustris. A, Petiole elongation rates under submerged and drained control conditions. Submergence started at t = 0. Growth rates were calculated every hour from length data monitored by linear variable displacement transducers. Black bars indicate 8-h dark periods. B, Petiole elongation rates over 3 d of drained control or submerged conditions without or with 1-MCP pretreatment to block ethylene perception. C, Petiole elongation rates under submerged and drained control conditions after pretreatment with 1-MCP. The submergence treatment started at t = 0. Growth rates were calculated every hour from length data monitored by linear variable displacement transducers. Black bars indicate 8-h dark periods. D, Petiole elongation in response to different exogenous ethylene concentrations (insert left: for comparison, petiole elongation rates over 3 d under drained and submerged conditions [black bar: fast accession; white bar: slow accession]). All data are mean ± se, n = 6 for A to C, n = 4 for D. For clarity reason, se is not shown in A and C. se varies from 2.9 × 10−3 mm h−1 to 9.7 × 10−2 mm h−1 (A) or 1.3 × 10−3 mm h−1 to 9.6 × 10−2 mm h−1 (C). Different letters indicate significant differences (Games-Howell test for B and Tukey test for D, P < 0.05). For statistics of the line charts in A and C, see Supplemental Table S1.
Carbohydrates Are Not Limiting in Either Accession
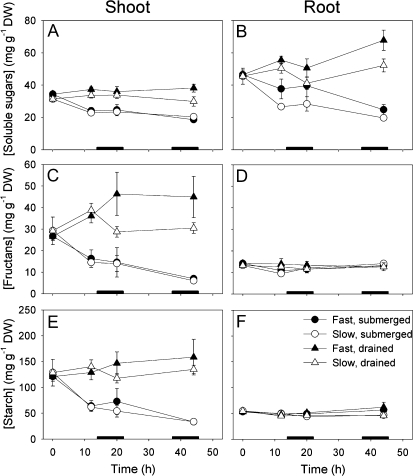
Low levels of carbohydrates in R. palustris can hamper elongation growth under water (Groeneveld and Voesenek, 2003). Due to low rates of underwater photosynthesis in R. palustris (Mommer et al., 2005a, 2005b), the production of carbohydrates during submergence is very limited. Therefore, petiole elongation depends strongly on carbohydrates stored over the growth period before the onset of submergence. In our study, the shoots of slow and fast accessions had similar soluble sugars, fructans, and starch contents at the start of submergence, and consumed them at comparable rates, resulting in similarly reduced levels after 2 d of submergence. Roots of both accessions showed no decline in starch and fructans upon submergence, whereas a decline in root soluble sugars was observed, being stronger in the slow accession (Fig. 2). We conclude for R. palustris accessions that carbohydrate levels are not limiting the elongation rate of the slow accession and thus that carbohydrates cannot account for the differences in flooding-induced petiole elongation between the two accessions. In contrast to our data, flood-tolerant and intolerant cultivated rice lines did show different consumption rates of starch and soluble sugars during submergence treatments (Fukao et al., 2006).
Figure 2.
Concentrations of soluble sugars (A and B), fructans (C and D), and starch (E and F) of the shoots (A, C, and E) and roots (B, D, and F) of the slow (white symbols) and fast (black symbols) accessions of R. palustris under submerged (circles) and drained control (triangles) conditions. The submergence treatment started at t = 0. Data are mean ± se, n = 4. Black bars indicate 8-h dark periods. For statistics, see Supplemental Table S2. DW, Dry weight.
Variation between Accessions Comes from an Ethylene-Controlled Pathway
Flooding-induced shoot elongation in wetland plants is regulated by the interplay between plant hormones and downstream targets that affect the cell wall structure (Voesenek et al., 2006). In R. palustris, flooding triggers petiole elongation by prompting a cellular increase in the gaseous hormone ethylene (Bailey-Serres and Voesenek, 2008). The ethylene action inhibitor 1-methylcyclopropene (1-MCP) strongly, but not completely, inhibited flooding-induced petiole elongation in both accessions (Fig. 1, B and C). This incomplete inhibition and the high specificity of 1-MCP for ethylene (Serek et al., 1994) suggest that ethylene-independent components contribute to petiole elongation in submerged R. palustris. This is consistent with earlier findings that submergence-induced growth rates usually exceed those obtained under ethylene fumigation (Fig. 1D; Voesenek et al., 1997). Interestingly, 1-MCP completely leveled off the differences in underwater growth rates between the two accessions (Fig. 1C), suggesting that an ethylene-controlled pathway is responsible for the variation in the rate of underwater elongation in R. palustris. Furthermore, the diurnal growth pattern as observed in untreated control plants (Fig. 1A) completely disappeared upon 1-MCP pretreatment (Fig. 1C), suggesting a role for ethylene in maintaining diurnal growth patterns in nonsubmerged R. palustris plants.
External ethylene application can mimic the difference in petiole elongation rates between the two accessions over a range of ethylene concentrations (Fig. 1D). This confirms that variation in submergence-induced elongation between Rumex accessions is regulated by an ethylene-dependent pathway.
Down-Regulation of ABA Levels Differs in Two Accessions
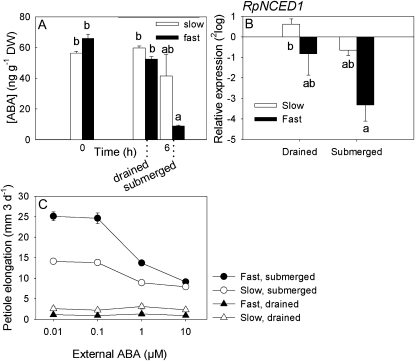
One of the first targets of accumulated ethylene in submerged plants is ABA (Hoffmann-Benning and Kende, 1992; Benschop et al., 2005; Fukao and Bailey-Serres, 2008). Experiments on R. palustris using the ethylene perception inhibitor 1-MCP showed that accumulation of ethylene is essential to induce a severe decrease of endogenous ABA levels (Benschop et al., 2005). Due to the combined inhibition of various biosynthesis genes belonging to the RpNCED family (Benschop et al., 2005) and the fast degradation of ABA (Benschop et al., 2005; Saika et al., 2007), ABA levels decline by almost 70% in 1 h (Benschop et al., 2005). The fast elongating R. palustris accession showed a much stronger decline in ABA levels after 6 h than did the slow elongating accession (Fig. 3A). This is accompanied by a stronger tendency toward down-regulation of RpNCED1 in the fast accession (Fig. 3B). These results suggest that differences in endogenous ABA determine the different elongation rates of submerged petioles of the two accessions. Supporting this finding is the observation that high levels of externally applied ABA, probably saturating ABA-mediated growth arrest in both accessions, eliminated the growth difference between fast and slow genotypes during submergence (Fig. 3C). These high ABA levels could not repress submergence-induced petiole elongation completely, suggesting that ABA at high levels was not simply toxic to these accessions (Fig. 3C). Consistent with our data, an elongating rice variety also showed a slightly stronger ABA reduction than a nonelongating variety (Hattori et al., 2009). However, other rice projects could not find differential control of ABA levels between elongating and nonelongating cultivars (Fukao and Bailey-Serres, 2008), suggesting that rice breeding programs have not always bred for flooding responses universally.
Figure 3.
ABA is differentially regulated upon submergence in the fast and slow accessions of R. palustris. A, ABA concentration of the third-oldest petiole of the two accessions under drained control (t = 0 and t = 6 h) and submerged (6 h) conditions. DW, Dry weight. B, Relative transcript abundance of the R. palustris NCED1 gene in the third-oldest petiole of the two accessions after 6 h of submerged and drained control conditions. Values are measured with real-time reverse transcription-PCR with tubulin as internal standard, relative to the value at t = 0 and 2log transformed. C, Elongation rates of the third-oldest petiole of the two accessions upon exposure to different concentrations of ABA under submerged and drained control conditions. Data are mean ± se, n = 5 to 6 for A, n = 3 for B, n = 10 for C. Different letters indicate significant differences (Games-Howell test, P < 0.05). For statistics of the data in C, see Supplemental Table S3.
Interestingly, previous work with Rumex acetosa, a species unable to increase petiole elongation upon submergence and ethylene treatments, also suggested a critical role for ABA in controlling underwater elongation among species (Benschop et al., 2005). In petioles of this species no significant decline in endogenous ABA was observed upon submergence. However, artificially lowering ABA levels using pretreatment of fluridone resulted in a 3-fold increase in the petiole elongation rate upon submergence. Pretreatment with 1-MCP could not inhibit this stimulated elongation in R. acetosa, consistent with the downstream position of ABA relative to ethylene (Benschop et al., 2005). Thus, a crucial conclusion from this work is that naturally occurring variation in submergence-induced petiole elongation occurs not only between but also within species. More importantly, this variation in plasticity occurs at the same hormone in the signaling pathway, suggesting that similar evolutionary pressures operate on genetic variation within and between species.
Two Accessions Differ in GA Responsiveness
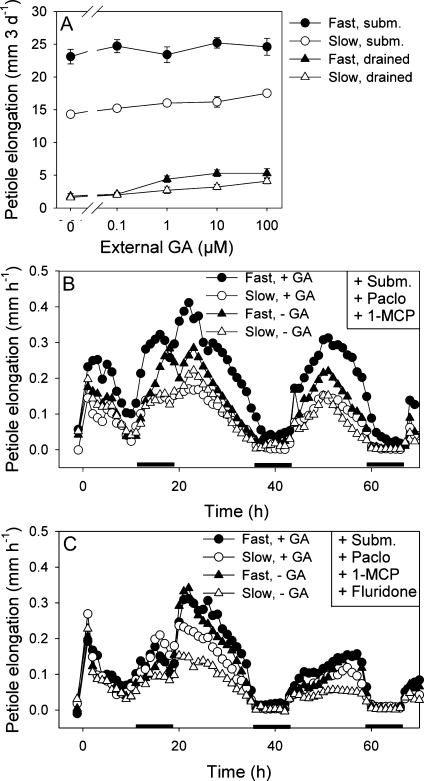
The central role of the plant hormone ABA in differentially controlling underwater growth rates in two accessions raises a question about the identity of the ABA targets. Previous work demonstrated that ABA inhibited expression of the GA biosynthesis gene RpGA3ox1 and thus the increase in GA biosynthesis upon submergence (Benschop et al., 2006). However, our data in Figure 4A show that high external GA additions cannot rescue the slow growing accession during submergence. Furthermore, the difference in flooding-induced petiole elongation between accessions remained when endogenous GA was reduced by a pretreatment with an inhibitor of GA biosynthesis (paclobutrazol; 10.3 mm 3 d−1 in the fast and 6.5 mm 3 d−1 in the slow accession in paclobutrazol pretreated and subsequently submerged plants, versus 21.8 and 16.8 mm 3 d−1, respectively, in submerged plants without paclobutrazol pretreatment; Supplemental Fig. S1). These data suggest that differences in flooding-induced elongation growth between the two accessions can, unexpectedly, not be explained by differences in endogenous GA that could have resulted from the differences in ABA content upon submergence.
Figure 4.
Responses to exogenous GA in the third-oldest petiole of the fast and slow accession of R. palustris. A, Dose-response curves for petiole elongation in response to different applied concentrations of GA in submerged (subm.) and drained control plants of the two accessions. Data are mean ± se, n = 10. For statistics, see Supplemental Table S4. B, Petiole elongation rates of the two accessions pretreated with paclobutrazol (Paclo; GA biosynthesis inhibitor) and 1-MCP (ethylene perception inhibitor) under submerged conditions with and without GA (10 mL 100 μm) in the submergence water. C, Petiole elongation rates of the two accessions pretreated with paclobutrazol, 1-MCP, and fluridone (ABA biosynthesis inhibitor) under submerged conditions with and without GA (10 mL 100 μm) in the submergence water. B and C, Submergence treatment started at t = 0. Growth rates were calculated every hour from length data monitored by linear variable displacement transducers. Data are mean of six to seven biological replicates. For clarity reason, se is not shown in the figures. se varies from 4.9 × 10−4 mm h−1 to 7.4 × 10−2 mm h−1 in B, and from 4.1 × 10−4 mm h−1 to 9.4 × 10−2 mm h−1 in C. Black bars indicate 8-h dark periods.
It is known for R. palustris that ethylene stimulates the responsiveness of petioles to GA (Rijnders et al., 1997), but there were no indications so far that ABA would affect GA responsiveness in this species (Benschop et al., 2006). However, work with fast-elongating rice showed that ethylene enhances the responsiveness of internodes to GA via a reduction in endogenous ABA (Hoffmann-Benning and Kende, 1992). To study the impact of ABA on GA responsiveness in the two contrasting accessions we inhibited ethylene action and GA biosynthesis simultaneously in submerged plants. Presumably these plants had control (i.e. similar to nonsubmerged plants) levels of ABA. Under these conditions externally applied GA stimulated petiole growth in the fast accession, but not in the slow one (Fig. 4B). When we lowered the ABA content by adding fluridone, GA responsiveness in the slow accession was restored, whereas the fast one elongated, irrespective of GA addition, with high rates similar to GA-treated plants in the experiment without fluridone (Fig. 4C). We conclude from these results that ABA controls variation in flooding-induced shoot elongation via GA responsiveness and that GA-induced petiole elongation is more repressed in the slow accession by relatively high ABA levels compared to the fast accession.
The use of chemical inhibitors of plant hormone biosynthesis, such as done here since genetic approaches are not possible in this nonmodel system, inevitably brings along the risk of side effects, including toxicity. It is for this reason that well-established inhibitors were used here, whose biochemical effects are known. However, since possible side effects cannot always be completely excluded, experimental design was such that data interpretation should not suffer. For example, application of fluridone, an inhibitor of carotenoid biosynthesis that suppresses ABA biosynthesis, has been shown previously to stimulate, rather than inhibit, elongation growth in both R. palustris and R. acetosa (Benschop et al., 2005). If fluridone would have major side effects, including toxicity, a growth reduction rather than stimulation should be expected. Triazole derivates that inhibit GA biosynthesis such as the classic inhibitors uniconazole, tetcyclacis, and paclobutrazol can also potentially inhibit P450s that have ABA 8′-hydroxylase activity, which might potentially lead to reduced ABA catabolism and therefore increased ABA levels (Kitahata et al., 2005; Saito et al., 2006). Within this class of inhibitors, however, particularly uniconazole and tetcyclacis have as strong effect on ABA 8′-hydroxylase activity, whereas for paclobutrazol this effect is much less severe. We therefore expect that if paclobutrazol inhibited ABA catabolism in our experiments, the effect will have been relatively mild. Furthermore, our experiments, shown in Figure 4, B and C, were designed such that a potential side effect of paclobutrazol on ABA catabolism is unlikely to lead to a misinterpretation of the ABA-GA interaction in Rumex. In Figure 4B, ABA levels are assumed to be at control (high) levels due to the application of 1-MCP (Benschop et al., 2005). A possible effect of paclobutrazol on ABA catabolism, leading to higher ABA levels, should not affect the outcome of this experiment since the ABA levels are already high. In Figure 4C, pretreatment with fluridone, in addition to 1-MCP and paclobutrazol, will lead to very low endogenous ABA levels in both accessions. Thus, although it cannot be ruled out that paclobutrazol would have some effect on ABA catabolism, in this study the effects observed are most likely related to its well-established reduction of GA biosynthesis.
It will be a topic for future studies to elucidate at exactly which level GA responsiveness is controlled. In rice, SUB1A controls stem elongation via DELLA protein levels (Fukao and Bailey-Serres, 2008). It is therefore possible that DELLA protein levels differ between both Rumex accessions during submergence. As shown for Arabidopsis this in turn can increase ABA levels via the XERICO genes (Ko et al., 2006; Zentella et al., 2007), thus potentially explaining the difference in ABA content between the two Rumex accessions upon submergence.
It is unlikely that the difference between the two accessions occurs at the very downstream level of cellular growth regulation, since the submergence-associated RpEXPA1 gene (Vreeburg et al., 2005) was not regulated differently between the accessions (Supplemental Fig. S2).
CONCLUSION
In summary, our experiments suggest that the variation in petiole elongation rates of submerged R. palustris accessions is regulated by an ethylene-controlled pathway that affects the dynamics of endogenous ABA concentrations in Rumex petioles upon submergence. Variation in endogenous ABA levels then affect the responsiveness to GA and thus the rate of underwater petiole elongation (Fig. 5). The slow elongating accession retains a relatively high ABA concentration, which then leads to a limited GA responsiveness and thus slower growth. First of all, this effect of ABA on GA responsiveness reveals a novel role of ABA regulating GA in the well-studied model species R. palustris. More importantly, if we compare this work with the earlier observed contrasting ABA levels in R. acetosa (slow elongating species) and R. palustris (faster elongating species), the results strongly suggest that differences within and between species in flooding-induced petiole elongation are regulated via the exact same pathways and switch points, i.e. by regulation of ABA levels and the subsequent GA responses. This makes it likely that due to the strong selective force of flooding stress identical evolutionary processes have been acting on genotypic variation within and between species in this group of wetland plant species.
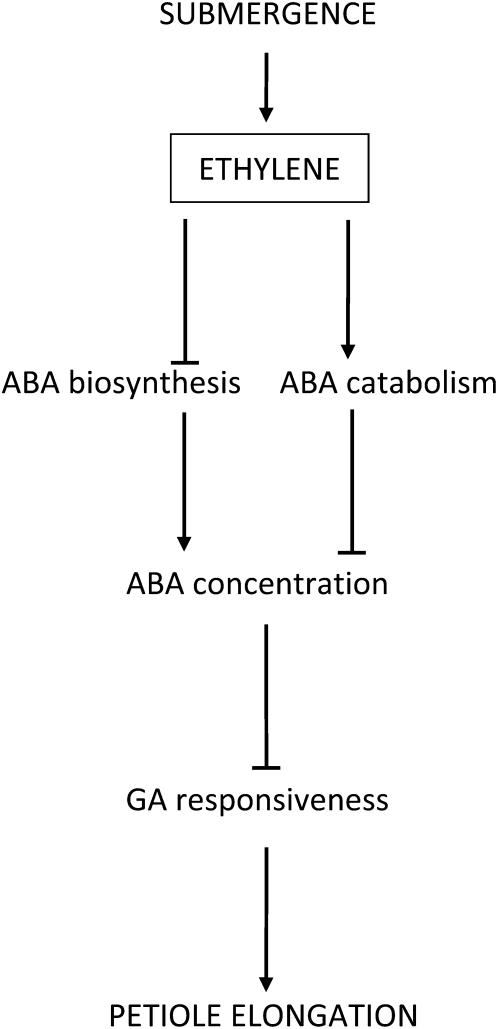
Figure 5.
Schematic presentation of the signaling pathway in which submergence induces enhanced petiole elongation in Rumex accessions. Submergence causes accumulation of ethylene inside plant tissues. These elevated ethylene levels induce reduction of ABA biosynthesis and a stimulation of ABA catabolism (Benschop et al., 2005) and lead to a lower endogenous ABA concentration. This stimulates GA signaling and ultimately enhances petiole elongation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Two representative accessions of Rumex palustris Sm. were selected from Chen et al. (2009) with a contrasting petiole elongation in 7 d complete submergence. The fast accession is from a river floodplain (Ewijk, The Netherlands) and showed strong petiole elongation, whereas the slow accession is from an artificial lake bank (Oostvoorne, The Netherlands) and showed relatively modest elongation upon submergence.
Seeds for the various experiments were germinated on filter paper moistened with tap water in petri dishes in a germination cabinet for 10 d (12 h light, 70 μmol m−2 s−1 photosynthetically active radiation, 25°C, and 12 h dark, 10°C). Morphologically similar seedlings were transplanted singly into plastic pots (70 mL), containing a mixture of potting soil and sand (2:1, v:v), enriched with 0.14 mg MgOCaO (17%; Vitasol BV) per pot. Before transplanting, each pot was given 20 mL nutrient solution containing: 7.5 mm (NH4)2SO4, 15.0 mm KH2PO4, 15.0 mm KNO3, 86 μm Fe-EDTA, 4.3 μm MnSO4, 1.8 μm ZnSO4, 0.32 μm CuSO4, 42 μm H3BO3, 0.53 μm Na2MoO4, and saturated with tap water. All chemicals were analytical reagents (Merck). Pots with seedlings were kept in trays covered with glass plates for 2 d after transplanting to prevent dehydration, after which they were placed on two layers of irrigation mat (Maasmond-Westland). The mats were automatically watered twice a day with tap water to saturation, and the excess water was drained away. Plants were grown for 17 d after transplanting in a growth chamber (20°C, 70% relative humidity, 16 h light: 200 μmol m−2 s−1 photosynthetically active radiation [Philips, HPI 400 W]) until the fifth-oldest leaf emerged (Banga et al., 1997). Just before experimentation plants were selected for homogeneity of developmental stage of the youngest leaf.
Plant Growth Measurements
The length of the third-oldest petiole was measured at the start and the end of an experiment to the nearest millimeter using a ruler. To monitor growth kinetics of this petiole, elongation was measured using linear variable displacement transducers (Schlumberger Industries; type ST 2000) according to Voesenek et al. (2003), adjusted with clamps designed to measure elongation of only the petioles and with a net pulling weight of 5 g. Plants were placed singly into the transducer setup with the junction between petiole and lamina attached to the clamps. Length of the petiole was recorded every 10 s and growth rates of the petioles were calculated by fitting lines through intervals of 1 h before and after the start of treatments.
Submergence Treatments
During submergence treatments, plants were placed singly in open-top glass cuvettes (diameter: 8.3 cm, height: 23.8 cm) and connected to the transducer setup. To achieve complete submergence, demineralized water was gently added into the cuvettes from the bottom until the cuvettes were full. Control plants were put in the cuvettes, but not submerged. For submergence treatments without transducer measurements, plants were fully submerged in larger open-top white plastic containers (60 × 40 × 27 cm) with demineralized water. Control plants rested on the irrigation mats. The topsoil layer of submerged plants was removed before experiments to prevent algal growth during the submergence treatment. The submergence treatments lasted from 6 h to 3 d in various experiments.
Application of Chemicals
GA3 (Duchefa), ABA (Sigma), and paclobutrazol (Duchefa) were dissolved in 96% ethanol, and fluridone (Fluka) in acetone, to various stock concentration and diluted with demineralized water 1,000 times to various final concentrations. Paclobutrazol (10 mL 100 μm) was given to plants via the soil 4 d before the start of an experiment, and fluridone (10 mL 100 μm) 3 d. GA or ABA was added to the flood water at various final concentrations. For control plants, 10 mL 0.1% (v:v) ethanol or acetone was given. Ethylene was applied to air-grown plants in a flow-through setup with a flow rate of 4 L min−1 at different concentrations. 1-MCP was applied for 3 h and 1 d before submergence to plants in closed containers at a concentration of 1 μL L−1 and on the day of submergence. This double pretreatment was to ensure optimal effectiveness for ethylene inhibition.
Nonstructural Carbohydrate Measurements
Complete shoots and roots were harvested after submergence or drained control treatments, frozen in liquid nitrogen immediately, and kept at −80°C before freeze drying. After freeze drying, shoot and root weights were measured, and the tissues were ground separately to pass a 0.08 mm sieve. Accurately weighed (around 10 mg) homogeneous powder of shoot or roots was used for nonstructural carbohydrate measurements. As a first step, an ethanol extraction was performed in two rounds at 25°C. After centrifugation, the supernatant was cleaned up by two rounds of chloroform additions and finally used for determination of soluble sugars. The residue from the ethanol extraction was dissolved in water at 60°C and subsequently centrifuged. The new supernatant contained fructans that were subsequently determined. The residue from this step was dried and subsequently boiled with HCl to extract starch. The concentration of the three fractions of carbohydrates was determined using the anthrone color reaction (Yemm and Willis, 1954). Anthrone reagent (2.5 mL; containing 0.04 mm anthrone, 6% ethanol, and 75% H2SO4) was added to 100 μL sample per fraction, and incubated in a boiling water bath for 7.5 min, after which it was cooled down immediately in ice water. The absorbance was measured using a spectrophotometer (Hitachi U-2000, Goffin Meyvis) at 625 nm. Concentrations of sugars were calculated using a calibration curve based on solutions containing known amounts of Glc covering the range of samples’ absorbance.
Endogenous ABA Measurements
The third-oldest petioles were harvested after submergence or drained control treatments, frozen in liquid nitrogen immediately, and kept at −80°C before freeze drying. Thirty petioles were pooled to have enough material per sample. The quantification of ABA and ABA metabolites were carried out by the Plant Biotechnology Institute, National Research Council Canada using HPLC electrospray ionization mass spectroscopy/mass spectroscopy. For a detailed description of the method used, see Chiwocha et al. (2003).
RNA Extraction and Real-Time Reverse Transcription-PCR
The third-oldest petioles were harvested after submergence or drained control treatments, frozen in liquid nitrogen immediately, and kept at −80°C before extraction. Five petioles were pooled to have enough material per sample. RNA was extracted using a modified method of Kiefer et al. (2000). Nucleon Phyto Pure extraction resin (50 μL, GE Healthcare) was used. Residual genomic DNA was broken down with two to six times of treatments of RNase-free DNaseI (Applied Biosystems). Approximately 1 μg of total RNA was used for cDNA synthesis using random hexamers (Invitrogen) and the SuperScript III Reverse Transcriptase kit (Invitrogen). Reverse transcription-PCR was performed with a Bio-Rad MyiQ single-color real-time PCR detection system with SYBR green as fluorescent intercalating dye (Bio-Rad). Primer sequences are 5′-CCATACTCCACCAGTGTTGC-3′ and 5′-CATCATCATCACCCTCAACG-3′ for tubulin, 5′-TTCTCCGGCCAGCTCAACT-3′ and 5′-CGAACATTTCTTTGGTGACGG-3′ for RpNCED1, and 5′-AGACGTTCACTCGGTGTCGAT-3′ and 5′-CAGTTCTGGCCCCAATTCC-3′ for RpEXPA1. Relative mRNA values were calculated using the comparative 2−ΔΔCt method described by Livak and Schmittgen (2001), expressing mRNA values relative to tubulin RNA. All transcript levels were presented relative to the value obtained at t = 0 h and 2log transformed.
Statistical Analyses
We performed ANOVA followed by Tukey/Games-Howell post hoc comparisons for specific comparisons among groups (SAS version 9.1; SPSS version 16). Transformation was used when necessary to improve the homogeneity of variance.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ173535 (RpNCED1) and AF167360.1 (RpEXPA1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Submergence-induced petiole elongation with and without the GA biosynthesis inhibitor paclobutrazol in R. palustris.
Supplemental Figure S2. Relative transcript abundance of RpEXPANSIN A-1 in petioles of control and submerged R. palustris plants.
Supplemental Table S1. ANOVA table from statistical analyses of data presented in Figure 1.
Supplemental Table S2. ANOVA table from statistical analyses of data presented in Figure 2.
Supplemental Table S3. ANOVA table from statistical analyses of data presented in Figure 3.
Supplemental Table S4. ANOVA table from statistical analyses of data presented in Figure 4.
Supplementary Material
Acknowledgments
We thank Rob Welschen and Yvonne de Jong-van Berkel for technical assistance and Julia Bailey-Serres for commenting on a draft version of this manuscript. This is part of a project from the Centre for Wetland Ecology, a partnership of The Netherlands Institute of Ecology, Radboud University Nijmegen, Utrecht University, and the University of Amsterdam.
References
- Bailey-Serres J, Voesenek LACJ. (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Banga M, Bögemann GM, Blom CWPM, Voesenek LACJ. (1997) Flooding resistance of Rumex species strongly depends on their response to ethylene: rapid shoot elongation or foliar senescence. Physiol Plant 99: 415–422 [Google Scholar]
- Bartelheimer M, Gowing D, Silvertown J. (2010) Explaining hydrological niches: the decisive role of below-ground competition in two closely related Senecio species. J Ecol 98: 126–136 [Google Scholar]
- Benschop JJ, Bou J, Peeters AJM, Wagemaker N, Guhl K, Ward D, Hedden P, Moritz T, Voesenek LACJ. (2006) Long-term submergence-induced elongation in Rumex palustris requires ABA-dependent biosynthesis of GA1. Plant Physiol 141: 1644–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Jackson MB, Guhl K, Vreeburg RAM, Croker SJ, Peeters AJM, Voesenek LACJ. (2005) Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J 44: 756–768 [DOI] [PubMed] [Google Scholar]
- Chen X, Huber H, de Kroon H, Peeters AJM, Poorter H, Voesenek LACJ, Visser EJW. (2009) Intraspecific variation in the magnitude and pattern of flooding-induced shoot elongation in Rumex palustris. Ann Bot (Lond) 104: 1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross ARS, Kermode AR. (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 3: 405–417 [DOI] [PubMed] [Google Scholar]
- Cho HT, Kende H. (1997a) Expression of expansin genes is correlated with growth in deepwater rice. Plant Cell 9: 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Kende H. (1997b) Expansins and internodal growth of deepwater rice. Plant Physiol 113: 1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Lee Y, Cho HT, Kende H. (2003) Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell 15: 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26: 17–36 [Google Scholar]
- Colmer TD, Voesenek LACJ. (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36: 665–681 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellins responses in rice. Proc Natl Acad Sci USA 105: 16814–16819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18: 2021–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld HW, Voesenek LACJ. (2003) Submergence-induced petiole elongation in Rumex palustris is controlled by developmental stage and storage compounds. Plant Soil 253: 115–123 [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H. (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H, Jacobs E, Visser EJW. (2009) Variation in flooding-induced morphological traits in natural populations of white clover (Trifolium repens) and their effects on plant performance during soil flooding. Ann Bot (Lond) 103: 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H, Kane NC, Heschel MS, von Wettberg EJ, Banta J, Leuck AM, Schmitt J. (2004) Frequency and microenvironmental pattern of selection on plastic shade-avoidance traits in a natural population of Impatiens capensis. Am Nat 163: 548–563 [DOI] [PubMed] [Google Scholar]
- Jackson MB. (2008) Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot (Lond) 101: 229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Ishizawa K, Ito O. (2009) Evolution and mechanisms of plant tolerance to flooding stress. Ann Bot (Lond) 103: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddy PA. (1992) Assembly and response rule: two goals fore predictive community ecology. J Veg Sci 3: 157–164 [Google Scholar]
- Kende H, van der Knaap E, Cho HT. (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiol 118: 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer E, Heller W, Ernst D. (2000) A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol Biol Rep 18: 33–39 [Google Scholar]
- Kitahata N, Saito S, Miyazawa Y, Umezawa T, Shimada Y, Min YK, Mizutani M, Hirai N, Shinozaki K, Yoshida S, et al. (2005) Chemical regulation of abscisic acid catabolism in plants by cytochrome P450 inhibitors. Bioorg Med Chem 13: 4491–4498 [DOI] [PubMed] [Google Scholar]
- Ko JH, Yang SH, Han KH. (2006) Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J 47: 343–355 [DOI] [PubMed] [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK. (1970) Stimulation of rice coleoptiles growth by ethylene. Planta 90: 333–339 [DOI] [PubMed] [Google Scholar]
- Lenssen JPM, de Kroon H. (2005) Abiotic constraints at the upper boundaries of two Rumex species on a freshwater flooding gradient. J Ecol 93: 138–147 [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Métraux JP, Kende H. (1984) The cellular basis of the elongation response in submerged deep-water rice. Planta 160: 73–77 [DOI] [PubMed] [Google Scholar]
- Mommer L, de Kroon H, Pierik R, Bögemann GM, Visser EJW. (2005a) A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytol 167: 197–206 [DOI] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJW. (2005b) Submergence-induced morphological, anatomical and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol 139: 497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave A, Jackson MB, Ling E. (1972) Callitriche stem elongation is controlled by ethylene and gibberellin. Nature 238: 93–96 [Google Scholar]
- Pierik R, van Aken JM, Voesenek LACJ. (2009) Is elongation-induced leaf emergence beneficial for submerged Rumex species? Ann Bot (Lond) 103: 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. (1984) Effect of submergence on translocation, starch content and amylolytic activity in deep water rice. Planta 162: 556–559 [DOI] [PubMed] [Google Scholar]
- Ridge I. (1987) Ethylene and growth control in amphibious plants. Crawford RMM, , Plant Life in Aquatic and Amphibious Habitats. Blackwell, Oxford, pp 53–76 [Google Scholar]
- Rijnders JGHM, Yang YY, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, Voesenek LACJ. (1997) Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 203: 20–25 [Google Scholar]
- Saika H, Okamoto M, Miyoshi K, Kushiro T, Shinoda S, Jikumaru Y, Fujimoto M, Arikawa T, Takahashi H, Ando M, et al. (2007) Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol 48: 287–298 [DOI] [PubMed] [Google Scholar]
- Saito S, Okamoto M, Shinoda S, Kushiro T, Koshiba T, Kamiya Y, Hirai N, Todoroki Y, Sakata K, Nambara E, et al. (2006) A plant growth retardant, Uniconazole, is a potent inhibitor of ABA catabolism in Arabidopsis. Biosci Biotechnol Biochem 70: 1731–1739 [DOI] [PubMed] [Google Scholar]
- Serek M, Sisler EC, Reid MS. (1994) Novel gaseous ethylene binding inhibitor prevents ethylene effects in potted flowering plants. J Am Soc Hortic Sci 119: 1230–1233 [Google Scholar]
- Silvertown J, Dodd ME, Gowing DJG, Mountford JO. (1999) Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400: 61–63 [Google Scholar]
- Sultan SE. (2007) Development in context: the timely emergence of eco-devo. Trends Ecol Evol 22: 575–582 [DOI] [PubMed] [Google Scholar]
- van Eck WHJM, van de Steeg HM, Blom CWPM, de Kroon H. (2004) Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos 107: 393–405 [Google Scholar]
- Vervuren PJA, Blom CWPM, de Kroon H. (2003) Extreme flooding events on the Rhine and survival and distribution of riparian plant species. J Ecol 91: 135–146 [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. (2009) Genetics of high-rise rice. Nature 460: 959–960 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. (2006) How plants cope with complete submergence. New Phytol 170: 213–226 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Jackson MB, Toebes AHW, Huibers W, Vriezen WH, Millenaar FF. (2003) De-submergence-induced ethylene production in Rumex palustris: regulation and ecophysiological significance. Plant J 33: 341–352 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Perik PJM, Blom CWPM, Sassen MMA. (1990) Petiole elongation in Rumex species during submergence and ethylene exposure: the relative contributions of cell division and cell expansion. J Plant Growth Regul 9: 13–17 [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, van de Steeg HM, de Kroon H. (2004) Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology 85: 16–27 [Google Scholar]
- Voesenek LACJ, Vriezen HW, Smekens MJE, Huitink FHM, Bögemann GM, Blom CWPM. (1997) Ethylene sensitivity and response sensor expression in petioles of Rumex species at low oxygen and high carbon dioxide concentrations. Plant Physiol 114: 1501–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer TD, Ammerlaan AHM, Staal M, Elzenga TM, Staals RHJ, Darley CP, McQueen-Mason SJ, et al. (2005) Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant J 43: 597–610 [DOI] [PubMed] [Google Scholar]
- Wilczek AM, Roe JL, Knapp MC, Cooper MD, Lopez-Gallego C, Martin LJ, Muir CD, Sim S, Walker A, Anderson J, et al. (2009) Effects of genetic perturbation on seasonal life history plasticity. Science 323: 930–934 [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance in rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Yang SH, Choi D. (2006) Characterization of genes encoding ABA 8′-hydroxylase in ethylene-induced stem growth of deepwater rice (Oryza sativa L.). Biochem Biophys Res Commun 350: 685–690 [DOI] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ. (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57: 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, et al. (2007) Global analysis of DELLA direct targets in early gibberellins signaling in Arabidopsis. Plant Cell 19: 3037–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.