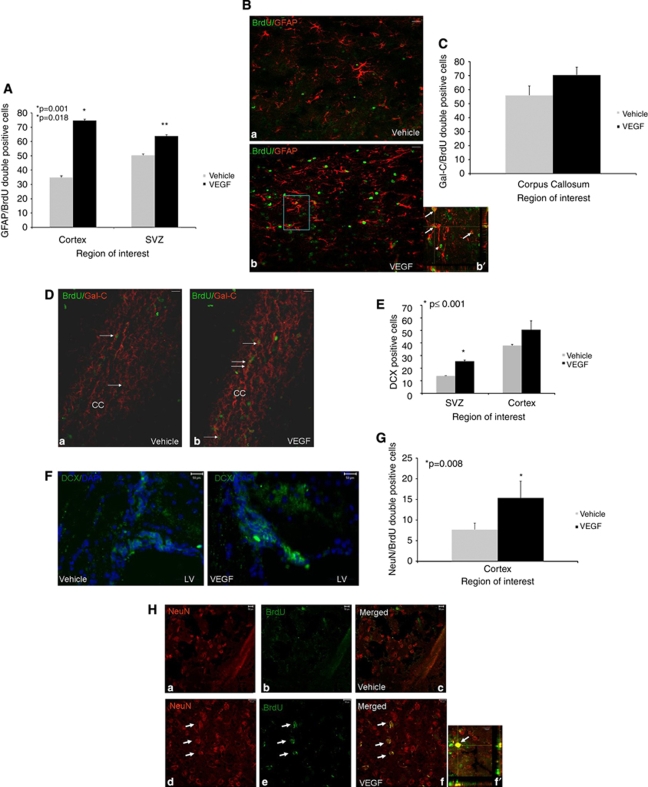
Figure 4.
Effects of vascular endothelial growth factor (VEGF) on newborn cell differentiation. Animals underwent closed head injury (CHI) and were killed 90 days later. Newborn cell fate was determined with immunohistochemistry. Most newborn cells in animals treated with VEGF differentiated into GFAP+ astrocytes in the perilesion cortex (A, B) and at the subventricular zone (SVZ), and the number of newborn astrocytes was significantly larger in these areas in VEGF-treated mice. The inset in (b′) is a Z section at × 400 taken from the rectangle in (B) showing colocalization of 5-bromo-2-deoxyuridine (BrdU) and GFAP in many of the positive cells (white arrows). Arrowheads point to BrdU+ GFAP− cells. At the corpus callosum, most newborn cells differentiated into newborn Gal-C+ oligodendroglia (C), but the differences between the groups were not significant. (D) A photomicrograph ( × 200) from the corpus callosum area (CC in D) showing an abundance of BrdU (green) and Gal-C (red) double-positive cells (white arrows) in both vehicle- (a) and VEGF (b)-treated mice. Significantly more doublecortin-positive (DCX+) cells were detected at the SVZ and surrounding the damaged cortex in VEGF-treated mice (E). (F) Sample photomicrographs ( × 400) from the SVZ area of vehicle- or VEGF-treated mice showing an abundance of DCX+ cells (green) in VEGF-treated animals. The numbers of NeuN/BrdU double-positive newborn neurons were significantly larger in the cortical area surrounding the lesion of VEGF-treated mice (G). (H) A sample of photomicrographs ( × 400) showing that NeuN (red) and BrdU (green) double-positive cells in the cortex surrounding the lesion of VEGF- (white arrows in panels d–f) but not vehicle (panels a–c)-treated mice. (f′) A confocal Z section showing the colocalization of BrdU (green) and NeuN (red) in a newborn neuron at the lesion border (bar graph=10 μm). Bar graphs in all figures=50 μm unless otherwise stated.