Abstract
Brain activation provokes nonoxidative carbohydrate consumption and during exercise it is dominated by the cerebral uptake of lactate resulting in that up to ∼1 mmol/ 100 g of glucose equivalents cannot be accounted for by cerebral oxygen uptake. The fate of this ‘extra' carbohydrate uptake is unknown, but it may be that brain metabolism is balanced by a yet-unidentified substance(s). This study used a nuclear magnetic resonance-based metabolomics approach to plasma samples obtained from the brachial artery and the right internal jugular vein in 16 healthy young males to identify carbon species going to and from the brain. We observed a carbohydrate accumulation of 255±37 μmol/100 g glucose equivalents at exhaustion not accounted for by the oxygen uptake. Although the cumulated uptake was lower than earlier observed, the results show that glucose and lactate are responsible for the majority of the carbon exchange across the brain. Even during intense exercise associated with the largest nonoxidative carbohydrate consumption, the brain did not show significant release of any other metabolite. We conclude that during exercise, the surplus carbohydrate uptake by the brain cannot be accounted for by changes in the NMR-derived plasma metabolome across the brain.
Keywords: carbohydrate, lactate, metabolism, NMR
Introduction
Cerebral activation, including changes in electrical (Kristeva-Feige et al, 2002; Rasmussen et al, 2004) and sensory (Friedman et al, 1991) activity during physical exercise, increases the brain's uptake of carbohydrate out of proportion to its oxygen uptake, that is cerebral activation is intimately coupled to so-called nonoxidative carbohydrate consumption (Dalsgaard, 2006; Fox et al, 1988; Ide et al, 1999). At a low arterial lactate concentration glucose dominates cerebral substrate consumption, but as plasma lactate increases during intensified exercise, lactate becomes important (Dalsgaard, 2006; van Hall et al, 2009). The largest increase in blood lactate is seen during maximal whole-body exercise (Nielsen et al, 1999) and the resting ratio between the brain's uptake of oxygen to that of carbohydrate (glucose plus 1/2 lactate) of 6 may decrease to a nadir of 1.7 (Volianitis et al, 2008). During maximal exercise, the brain may take up ∼3.5 times more carbohydrate than can be accounted for by its oxygen uptake. Thus, during maximal exercise, this unaccounted-for uptake can reach ∼1 mmol/100 g of carbohydrate for the whole brain (10 to 15 mmol) or up to 40 to 50% of the total carbohydrate uptake (Quistorff et al, 2008).
As brain activation takes place repeatedly during the day, we considered that cerebral nonoxidative carbohydrate consumption could be reversed in the recovery from cerebral activation, including exercise. Surprisingly, however, in the many studies performed, including samples obtained 30 to 60 mins after exercise, no significant recovery of the oxygen-to-carbohydrate balance has been observed (Dalsgaard, 2006). Thus, it may be that brain metabolism is balanced by some yet-unidentified substance leaving the brain. Besides glucose and lactate uptake during exercise, the brain also takes up ketone bodies during fasting (Hasselbalch et al, 1994), whereas its uptake of free fatty acids, amino acids (Dalsgaard et al, 2002), pyruvate (Rasmussen et al, 2006), and citrate (Rasmussen et al, 2009) accounts for <3% of the surplus uptake.
Here, it is evaluated by a metabolomics approach to arterial and internal jugular venous blood samples whether the ‘surplus' uptake of carbohydrate, compared with the cerebral uptake of oxygen during intense exercise is compensated for by a release of carbon source(s) other than glucose and lactate. For this, the nuclear magnetic resonance (NMR) is used as a near universal detector of small organic substances. A substance released from the brain in a substantial amount should be directly detected in the spectra or revealed by spectral covariance with other metabolites important for the energy metabolism (Nicholson and Wilson, 1989).
Materials and methods
Sixteen males at an age of 20 to 34 years participated in this study (75±7 kg, 179±6 cm, maximal oxygen uptake 4.2±0.7 L/min; mean±s.d.). The subjects provided informed consent to the study as approved by the Ethics Committee of Copenhagen and Frederiksberg (KF 01257177).
Before the experiment, the subjects were familiarized with the protocol and performed incremental cycling (Monarc Ergomedic, Monarc, Sweden) to determine maximal oxygen uptake. On the day of the main study, a catheter was, under local anesthesia (2% lidocaine), placed retrograde with Seldinger technique in the right internal jugular vein (1.6 mm, 14 gauge; ES-04706, Arrow International, PA, USA) as guided by an ultrasound image and the catheter was advanced to the bulb of the vein. Arterial blood was drawn from a catheter in the brachial artery (1.1 mm, 20 gauge) of the nondominant arm. Mean arterial pressure was measured with a transducer (Edwards Life Sciences, Irving, CA, USA) placed at the level of the heart, interfaced with a Dialogue-2000 (IBC-Danica, Copenhagen, Denmark), sampled at 200 Hz (DI-720, Dataq, OH, USA), and stored.
Blood was sampled in pre-heparinized syringes, kept anaerobic, and analyzed within 30 mins for glucose, lactate, and oxygen content using an ABL 725 (Radiometer, Copenhagen, Denmark). Blood for metabolomic analysis was drawn into tubes containing ethylenediaminetetraacetic acid, kept anaerobic and cool, and spun (3500 r.p.m.; 15 mins) with plasma stored at −80°C until analyzed.
Changes in cerebral blood flow were evaluated by the transcranial ultrasound Doppler (TCD; Transcan, EME, Überlingen, Germany)-derived mean flow velocity (Vmean) in the middle cerebral artery (MCA). Depending on the position with the best signal-to-noise ratio, the proximal part of the MCA was insonated at a depth of 40 to 60 mm from the temporal bone and the probe was secured with a headband. Cerebral blood flow (CBF) was derived from the changes in MCA Vmean assuming an unchanged internal diameter of the MCA (Bradac et al, 1976; Serrador et al, 2000) with resting mean CBF set at 46 mL/100 g/min (Madsen et al, 1993).
To express cerebral nonoxidative carbohydrate consumption, the oxygen–glucose index (OGI) and the oxygen–carbohydrate index (OCI) were calculated as the ratio between the cerebral metabolic rate for oxygen and the cerebral metabolic rate for glucose and glucose+1/2 lactate, respectively (Dalsgaard et al, 2002; Ide et al, 1999). The OGI and OCI ratios were considered to be independent of CBF as flow would enter both the denominator and numerator. The cumulated cerebral uptake of glucose, lactate, O2, and CO2 was calculated from the arterial and internal jugular venous concentrations (Quistorff et al, 2008):
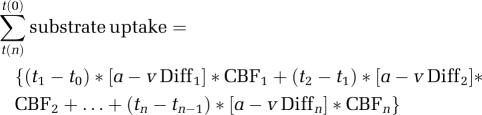 |
Similarly, the cumulated cerebral uptake balance between glucose, lactate, O2, and CO2 all normalized to glucose equivalents (glucose+1/2 lactate−1/6O2 and glucose+1/2 lactate+1/6 CO2) were calculated. These balances reflect the net uptake of glucose and lactate by the brain that cannot be accounted for by O2 uptake or CO2 release and, therefore, represents its nonoxidative carbohydrate consumption.
After instrumentation, the subjects rested for 1 h where the blood was drawn. The subjects then exercised for 20 mins at a light intensity (124±21 W) and blood for blood gas analysis was drawn before, after 10 mins, and at the end of exercise and also after 1, 3, and 5 mins of recovery, whereas blood for metabolomic analysis was drawn at the end of exercise. After 90 mins of recovery, the subjects completed graded exercise with a 10% increase in workload every 5th minute until exhaustion (279±75 W). Blood was drawn with the same protocol as during light exercise, except that the subjects were instructed to provide warning before they were exhausted to allow for blood sampling.
Preparation of Samples for Nuclear Magnetic Resonance Metabolomics
The plasma samples were kept at −80°C until analysis. After thawing at room temperature for ∼1 h, the samples were gently mixed, 600 μL drawn, and mixed with 50 μL D2O (D 99.8%, sterile). A measure of 600 μL was then transferred to single use NMR-tubes, refrigerated until analyzed overnight using a 25-position sample changer and in-house written control sequences. Most of the samples were analyzed twice with a second revolution of the sample changer. The analysis time (including sample transfer, temperature equilibration, gradient shimming, and acquisition with two different pulse sequences) was ∼40 mins.
Nuclear Magnetic Resonance Spectra
The NMR spectra were recorded at 10°C on a Bruker Avance DRX600 spectrometer (Billerica, MA, USA) equipped with a 5 mm inverse probe head with z-gradient. T2-filtered spectra (0.2 secs echo-time for the T2 filter) were recorded using a CPMG-sequence augmented with presaturation during the relaxation delay followed by a spoil gradient to attenuate the intensity of the water signal to 7 to 10 times the intensity of the anomeric signal from α-Glc. For each spectrum, 32 scans were added and 216 data points were acquired with a sweep width of 12 kHz and a pulse repetition time of 5.64 secs. The carrier position (locked to the D2O signal) was kept constant in all experiments.
Preparation of Data for Statistical Analysis
The spectra were Fourier transformed to 215 real data points after exponential multiplication with a line-broadening factor of 0.3 Hz. The zeroth order phase was automatically determined using phase correction routines in Topspin (Bruker Biospin, version 1.3) and manually adjusted when needed. The spectra were imported into Matlab (MathWorks Inc, MA, USA) using in-house routines and baseline corrected and calibrated using the doublet of the anomeric signals from α-Glc set to 5.23 p.p.m. and projected to a common axis corresponding to a range from 10 to 0.5 p.p.m. in 8,192 steps using cubic interpolation. The matrix was further reduced in size by integration over the total range, excluding the ranges of residual water signal ±0.2 p.p.m., and signals from free ethylenediaminetetraacetic acid and its Ca2+ and Mg2+ complexes at 3.60±0.06, 3.15±0.15, 2.67±0.03, and 2.55±0.05 p.p.m. (Nicholson et al, 1983). Buckets were selectively merged according to a scheme based on covariance of integrated values in vicinal buckets. Vicinal buckets with a correlation coefficient (R2) >0.80 were merged. In addition, vicinal buckets with a negative correlation coefficient were merged to cover those instances when a signal had small differences in chemical shift. Buckets with the smallest variances were subsequently excluded to provide 200 variables for each spectrum. The spectral data were normalized to the externally determined glucose concentrations and validated using a PLS model to predict lactate concentrations.
Statistical Analysis
For time-series data, one- and two-way repeated-measures ANOVA with the Tukey post hoc procedure identified statistical significances (P<0.05) in SAS (9.1, SAS Institute Inc., Cary, NC). Values are presented as mean (s.d.) in the text and as mean with s.e.m. in the figures. Metabolomic analysis was performed with PLS toolbox (version 5.0, Eigenvector Research inc., WA, USA) and Statistics Toolbox (version 7.1, MathWorks). Cross-validation was performed by calibrating models with data from a subset of subjects and predicting the response for the subset of data from an excluded subject. This was repeated until the response for all subjects were predicted. The numbers of latent variables in the models were determined by the root mean square error of cross-validation (rmsecv) value. Significant differences between the spectra medians were tested using Wilcoxon-rank sum test.
Results
Heart rate, blood pressure, arterial plasma concentrations, and arterio-venous differences (a-v Diff) for oxygen, glucose, and lactate are reported in Table 1. Resting upright on the bicycle arterial oxygen saturation was 98±1% and the internal jugular venous saturation 58±4%.
Table 1. Cardiovascular and cerebral metabolic variables after 20 mins of submaximal cycling (124±21 W) or after cycling exercise to exhaustion (279±75 W).
| Rest | Submaximal | Exhaustion | |
|---|---|---|---|
| HR (beat per minute) | 90±11 | 133±13*** | 164±13*** |
| MAP (mm Hg) | 93±5 | 102±5** | 100±5** |
| MCA Vmean (% of rest) | 100±0 | 112±12 | 107±19 |
| O2 a-v Diff (mmol) | 3.5±0.3 | 3.1±0.4* | 4.1±0.4*** |
| PaCO2 (kPa) | 5.0±0.5 | 5.0±0.4 | 3.8±0.4*** |
| PvCO2 (kPa) | 6.4±0.5 | 6.5±0.4 | 5.6±0.3*** |
| CO2 a-v Diff (mmol) | 3.6±0.3 | 3.6±0.3 | 4.4±0.2*** |
| Glca (mmol/l) | 5.8±0.3 | 5.5±0.3 | 5.7±0.4 |
| Laca (mmol/l) | 1.2±0.3 | 1.7±0.8 | 10.8±1.7*** |
| Glc a-v Diff (mmol/l) | 0.6±0.1 | 0.6±0.1 | 0.8±0.1** |
| Lac a-v Diff (mmol/l) | −0.1±0.1 | −0.1±0.1 | 0.8±0.2*** |
| OGI | 5.8±0.9 | 5.4±1.2 | 5.4±0.9 |
| OCI | 6.2±0.7 | 6.1±0.6 | 3.8±0.5*** |
HR, heart rate; MAP, mean arterial pressure; MCA Vmean, middle cerebral artery mean flow velocity; *, **, and ***, different from rest P<0.05, P<0.01, and P<0.001, respectively. Values are mean±s.d.
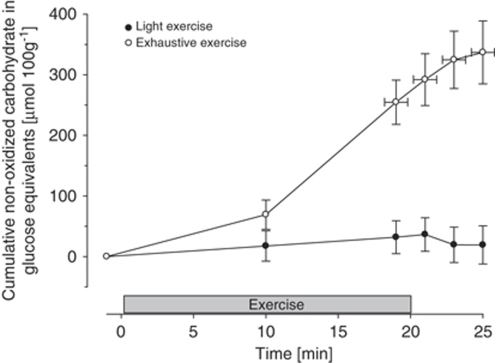
Light exercise (124 W) for 20 mins increased heart rate and blood pressure (Table 1) and decreased a-v Diff for oxygen (P<0.05), whereas no other changes in cerebral metabolism were observed. The time to exhaustion during graded exercise (279 W) was 20.1±3.2 mins. Heart rate increased compared with both rest and light exercise, whereas blood pressure was unchanged from light exercise (Table 1). Exhaustive incremental exercise increased the cerebral a-v Diff for glucose and lactate (P<0.001) and an increase in the a-v Diff for oxygen was also observed (P<0.001). Nevertheless, the OCI decreased from control (P<0.001), whereas no significant change in OGI was observed. The cumulated uptake of glucose, lactate, and oxygen over the exercise bout and the balanced sum (glucose+1/2 lactate−1/6 oxygen) indicated a surplus uptake of carbohydrate of 255±37 μmol/ 100 g at exhaustion (P<0.001 versus light exercise, Figure 1).
Figure 1.
Carbon uptake in glucose equivalents by the brain during cycling exercise. Cumulated uptake of glucose, lactate, and oxygen over the exercise bout and the balanced sum (glucose+1/2 lactate−1/6 O2) indicate a surplus uptake of carbohydrate during maximal exercise. Values are mean±s.e.m. for 16 subjects.
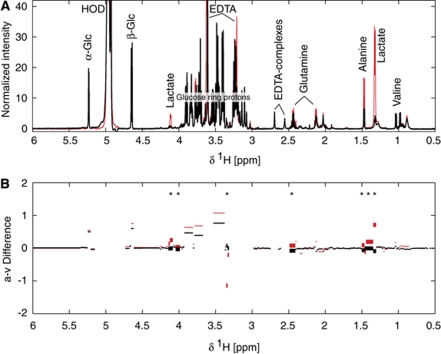
The acquired spectra were normalized to the determined glucose concentration and subsequently validated against the determined lactate concentrations. After the removal of 10 obvious outliers, a PLS model (with three latent variables) predicting the lactate concentration had a R2 of 0.87. Figure 2 shows selected NMR spectra at rest and at end of exhaustive exercise combined with median values of calculated differences between paired arterial and venous samples (a-v Diff). A few significant differences (P<0.05, two-sided Wilcoxon-rank sum test) between plasma spectrum samples at resting and exhaustive exercise were found. The data confirm the cerebral uptake of lactate (1.3 and 4.2 p.p.m.). The noise in the calculated differences makes the uptake of glucose not significant, but differences related to glucose (5.2, 4.6, 3.8 to 3.2 p.p.m.) are consistently higher at exhaustion compared with resting. Other significant differences observed were those for alanine and signals at 2.45 and 3.3 p.p.m. The latter one is negative, indicating a substance released from the brain during exercise, but looking into the original spectra, the signal seems to be too small to have any impact on the mass balance. The signal at 2.45 p.p.m. could be glutamine, but only one of the two visible signals displays a significant difference.
Figure 2.
NMR spectra (A) of arterial blood plasma sampled at rest (black) and at exhaustion (red). Median values (B) of the differences between integrated spectra of arterial and venous plasma (AV-difference) at rest (black) and at exhaustion (red). Significant differences between the medians, P<0.05 (Wilcoxon-rank sum test), are marked with stars and thicker lines. The NMR spectra were normalized to the externally determined concentration of glucose in the plasma samples.
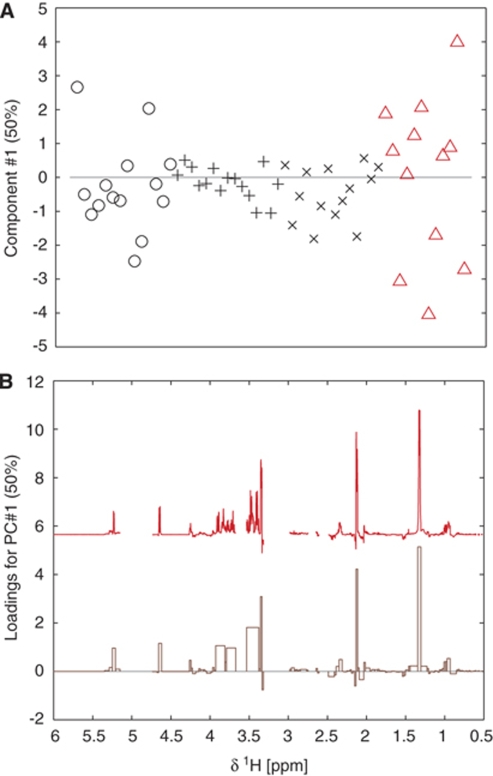
The matrix of differences between integrated spectral ranges of arterial and venous samples during exhaustive exercise was subjected to principal component analysis. Only one component, describing 50% of the variance in the dataset after mean centering, was significant according to cross-validation. The loadings (Figure 3B) reveal the positive correlation between aforementioned variables related to lactate and glucose. There is also one positive correlation with a signal at 2.1 p.p.m., which coincides with one of the signals ascribed to glutamine. Comparing the AV-differences during rest and exhaustive exercise (Figure 3A) reveals no clear trend for this principal component as expected. This can probably be attributed to that the underlying data is calculated from differences between integrated spectra and the added experimental variances because of this procedure.
Figure 3.
Scores (A) and loading (B) for the principal component analysis (PCA) of mean centered AV-differences for plasma sampled at rest (black, o, x), light exercise (black, +), and at exhaustion (red, Δ). The spectrum above the loading is the median spectrum of all spectra of samples from the exhaustive exercise scaled to fit the loading at respective variable and corresponding spectral range.
Thus, from the metabolomic data, we could not identify any component produced by the brain to balance the uptake of glucose and lactate. We observe no obvious release candidate from the brain as the calculated AV-differences were mostly positive.
Discussion
This study confirmed that low level dynamic exercise does not influence cerebral metabolism either expressed as CMRO2 or as OGI or OCI (Ide et al, 1999; Seifert et al, 2009). In addition, a marked increase in CMRO2 and similar marked increase in the cerebral nonoxidative metabolism are in accordance with what has been reported in earlier studies in this experimental setup, albeit we did not observe a decrease in OCI to same extent as reported during maximal rowing in well-trained athletes (Volianitis et al, 2008). Thus, the cumulated ‘surplus' carbohydrate uptake by the brain, the amount not explained by cerebral oxygen uptake, amounted to ∼250 μmol/100 g, whereas values of ∼1 mmol/100 g or ∼15 mmol for the whole brain has been calculated based on data from exhaustive ergometer rowing in highly trained competitive rowers (Quistorff et al, 2008).
The possibility of substance removal by a perivascular flow draining to the venous system down stream of the sampling point in the jugular vein could be considered (Ball et al, 2010). We find, however, that the magnitude of the perivascular flow must be 5 to 10% of the CBF to provide an explanation for the apparent surplus uptake. There are as yet no reports of any such perivascular drain from the human brain, and it thus remains to be tested whether perivascular flow in human beings during exercise can account for the surplus carbon uptake.
If we assume that cerebral CO2 tissue tension equilibrates with internal jugular venous CO2 tension (Table 1), the reduction in PvCO2 from rest to maximal exercise suggests that CO2 was washed out from the brain. Thus, it is unlikely that the reduction in OGI and OCI is explained by using CO2 rather than O2.
The main purpose of this study was to evaluate whether the large nonoxidative carbohydrate consumption observed during maximal exercise and, thereby, representing a much larger deviation in cerebral metabolism than developed in response to, for example, mental stress (Madsen et al, 1992) can be explained by export from the brain of some unknown substance. In confirmation of data based on a−v Diff (Dalsgaard, 2006) and evaluation by 13C-labeled lactate (van Hall et al, 2009), a metabolomic approach based on arterial and venous blood confirmed that exercise, and more so maximal exercise, is associated with brain uptake of lactate in addition to glucose. The NMR-based metabolomic analysis, however, did not reveal a significant export of carbon substances (e.g., carbohydrates or fat), which could explain brain nonoxidative metabolism, that is the reduction in OCI maintained during activation. According to this unbiased approach to cerebral metabolism and in confirmation of dedicated biochemical analysis (Dalsgaard, 2006), it seems that major deviations from normal brain metabolism needs to be understood within the realm of glucose, lactate, oxygen, and carbon dioxide.
The main element in driving cerebral nonoxidative metabolism during exercise is the lactate uptake increasing with its arterial concentration as exercise intensity increases while the increase in glucose uptake is smaller. Thus, lactate to some extent replaces glucose as substrate (Kemppainen et al, 2005; van Hall et al, 2009), albeit it is accepted that brain activation increases glucose uptake (Fox et al, 1988; Madsen et al, 1999). The fate of the lactate is, therefore, of special interest. According to an evaluation by MR and analysis of cerebrospinal fluid after exercise, there is no indication for accumulation of lactate in the brain (Dalsgaard et al, 2004). Accordingly, a 1-13C lactate tracer study shows that at moderate lactate concentrations almost all lactate is oxidized (van Hall et al, 2009). Whether this observation also holds at the high arterial lactate concentration during maximal exercise remains to be established. It should be remembered, however, that the actual oxygen consumption sets a limit to the maximal lactate oxidation, that is lactate+3O2=3CO2+3H2O, indicating that with an a−v Diff of oxygen of typically 3 mmol/L, no >1 mmol/L lactate may be oxidized.
Limitations
The difference between arterial and venous samples in the NMR evaluation was not as clear as expected from a biochemical analysis. This could be due to analytical variance in the spectral data, or changes in the composition of the samples between sampling and analysis, or lack of specificity in the data (overlap of several components). The currently used NMR method is also limited in that it detects mostly low-molecular protonated species. The NMR analysis is performed on plasma, and the amount of metabolites inside red blood cells is not evaluated.
Changes in CBF were appreciated with transcranial ultrasound Doppler technique. An important assumption in the interpretation of the Doppler velocity data is an unchanged internal diameter of the insonated vessel. CBF is regulated distally to the basal cerebral vessels (Bradac et al, 1976) and, hence, the MCA diameter would not be expected to change (Giller et al, 1993; Serrador et al, 2000). In support hereof, the increase in the MCA Vmean during exercise mirrors the increase in the carotid (Hellström et al, 1996) and spinal arteries (K Sato, personal communication). Further, an increase in MCA Vmean during exercise is supported by increased near-infrared spectroscopy-derived cerebral oxygenation (Ide et al, 1999) and paralleled by a 133Xe-washout determination of CBF (Jørgensen, 1995).
Conclusion
The brain takes up an extra amount of carbohydrate than accounted for by its oxygen uptake when activated with exercise, and when the carbon balance is calculated, this deviation in cerebral metabolism persists. Using an NMR-based metabolomic approach, we conclude that the surplus carbohydrate uptake by the brain cannot be accounted for by release of a low-molecular proton bearing substance and that major deviations from normal brain metabolism needs to be understood within the realm of glucose, lactate, and oxygen.
The authors declare no conflict of interest.
References
- Ball KK, Cruz NF, Mrak RE, Dienel GA. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J Cereb Blood Flow Metab. 2010;30:162–176. doi: 10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of intercranial arteries and veins in dependence of different CO2 tension. Neuroradiology. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK. Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab. 2006;26:731–750. doi: 10.1038/sj.jcbfm.9600256. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Ide K, Cai Y, Quistorff B, Secher NH. The intent to exercise influences the cerebral O(2)/carbohydrate uptake ratio in humans. J Physiol. 2002;540:681–689. doi: 10.1113/jphysiol.2001.013062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol. 2004;554:571–578. doi: 10.1113/jphysiol.2003.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Friberg L, Mitchell JH, Secher NH. Effect of axillary blockade on regional cerebral blood flow during static handgrip. J Appl Physiol. 1991;71:651–656. doi: 10.1152/jappl.1991.71.2.651. [DOI] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W, Loftus CM, Muizelaar JP. Cerebral arterial diameters during changes in blood-pressure and carbon-dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- Hasselbalch S, Knudsen G, Jakobsen J, Hageman L, Holm S, Paulson OB. Brain metabolism during short-term starvation in humans. J Cereb Blood Flow Metab. 1994;14:125–131. doi: 10.1038/jcbfm.1994.17. [DOI] [PubMed] [Google Scholar]
- Hellström G, Fischer-Colbrie W, Wahlgren NG, Jögestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- Ide K, Horn A, Secher N. Cerebral metabolic response to submaximal exercise. J Appl Physiol. 1999;87:1604–1608. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- Jørgensen LG. Transcranial Doppler ultrasound for cerebral perfusion. Acta Physiol Scand Suppl. 1995;625:1–44. [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P, Knuuti J. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568:323–332. doi: 10.1113/jphysiol.2005.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol. 2002;113:124–131. doi: 10.1016/s1388-2457(01)00722-2. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Holm S, Herning M, Lassen NA. Average blood flow and oxygen uptake in the human brain during resting wakefulness: a critical appraisal of the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1993;13:646–655. doi: 10.1038/jcbfm.1993.83. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Schmidt JF, Holm S, Jørgensen H, Wildschiødtc G, Christensen NJ, Friberg L, Vorstrup S, Lassen NA. Mental stress and cognitive performance do not increase overall level of cerebral O2 uptake in humans. J Appl Physiol. 1992;73:420–426. doi: 10.1152/jappl.1992.73.2.420. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Buckingham MJ, Sadler PJ. High resolution proton NMR studies of vertebrate blood and plasma. Biochem J. 1983;211:605–615. doi: 10.1042/bj2110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Wilson ID. High resolution proton magnetic resonance spectroscopy of biological fluids. Prog Nucl Magn Reson Spectrosc. 1989;21:449–501. [Google Scholar]
- Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol Heart Circ Physiol. 1999;277:H1045–H1052. doi: 10.1152/ajpheart.1999.277.3.H1045. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22:3443–3449. doi: 10.1096/fj.08-106104. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Madsen CA, Nielsen HB, Zaar M, Gjedde A, Secher NH, Quistorff B. Coupling between the blood lactate to pyruvate ratio and MCA Vmean at the onset of exercise in humans. J Appl Physiol. 2009;107:1799–1805. doi: 10.1152/japplphysiol.00468.2009. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Plomgaard P, Krogh-Madsen R, Kim YS, van Lieshout JJ, Secher NH, Quistorff B. MCA Vmean and the arterial lactate-to-pyruvate ratio correlate during rhythmic handgrip. J Appl Physiol. 2006;101:1406–1411. doi: 10.1152/japplphysiol.00423.2006. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Stie H, Nybo L, Nielsen B. Heat induced fatigue and changes of the EEG is not related to reduced perfusion of the brain during prolonged exercise in humans. J Therm Biol. 2004;29:731–737. [Google Scholar]
- Seifert T, Rasmussen P, Brassard P, Homann PH, Wissenberg M, Nordby P, Stallknecht B, Secher NH, Nielsen HB. Cerebral oxygenation and metabolism during exercise following three months of endurance training in healthy overweight males. Am J Physiol Regul Integr Comp Physiol. 2009;297:R867–76. doi: 10.1152/ajpregu.00277.2009. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Fabricius-Bjerre A, Overgaard A, Stromstad M, Bjarrum M, Carlsson C, Petersen N, Rasmussen P, Secher NH, Nielsen HB. The cerebral metabolic ratio is not affected by oxygen availability during maximal exercise in humans. J Physiol. 2008;586:107–112. doi: 10.1113/jphysiol.2007.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]