Abstract
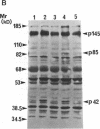
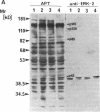
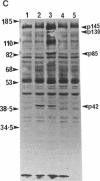
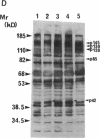
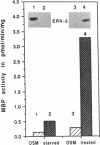
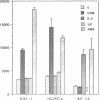
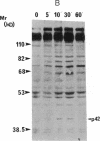
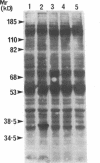
Oncostatin-M (OSM) is a potent mitogen for Kaposi's sarcoma (KS) cells. We studied signaling by the OSM receptor in three AIDS-related KS lines and show induction of tyrosine phosphorylation of 145-, 120-, 85-, and 42-kD substrates. The 42-kD substrate was identified as p42MAPK (mitogen-activated protein kinase), also known as ERK-2. This serine/threonine kinase relays mitogenic signals from receptor tyrosine protein kinases (TPKs) or receptor-associated TPKs to transcriptional activators. The OSM dose dependence for MAP kinase activation and induction of KS cell growth were almost identical, suggesting functional linkage. MAP kinase activation was dependent on tyrosine phosphorylation, and both OSM-induced MAP kinase activity and KS cell growth could be suppressed by TPK inhibitors, genistein and geldanomycin. OSM also stimulated tyrosine phosphorylation of similar substrates and MAP kinase activity in human vein endothelial cells. While it has been proposed that the OSM receptor may include the gp130 subunit of the IL-6 receptor and alpha-chain of leukemia inhibitory factor (LIF) receptor, neither LIF nor r.IL-6 induced tyrosine protein phosphorylation or p42MAPK activation in KS cells. However, r.IL-6 did stimulate tyrosine phosphorylation and p42MAPK activity in the human B cell line, AF-10, while OSM and LIF exerted no effects. Our results indicate that, although the OSM and IL-6 receptors share a common signaling pathway, this pathway is selectively activated by OSM in Kaposi's cells.
Full text
PDF

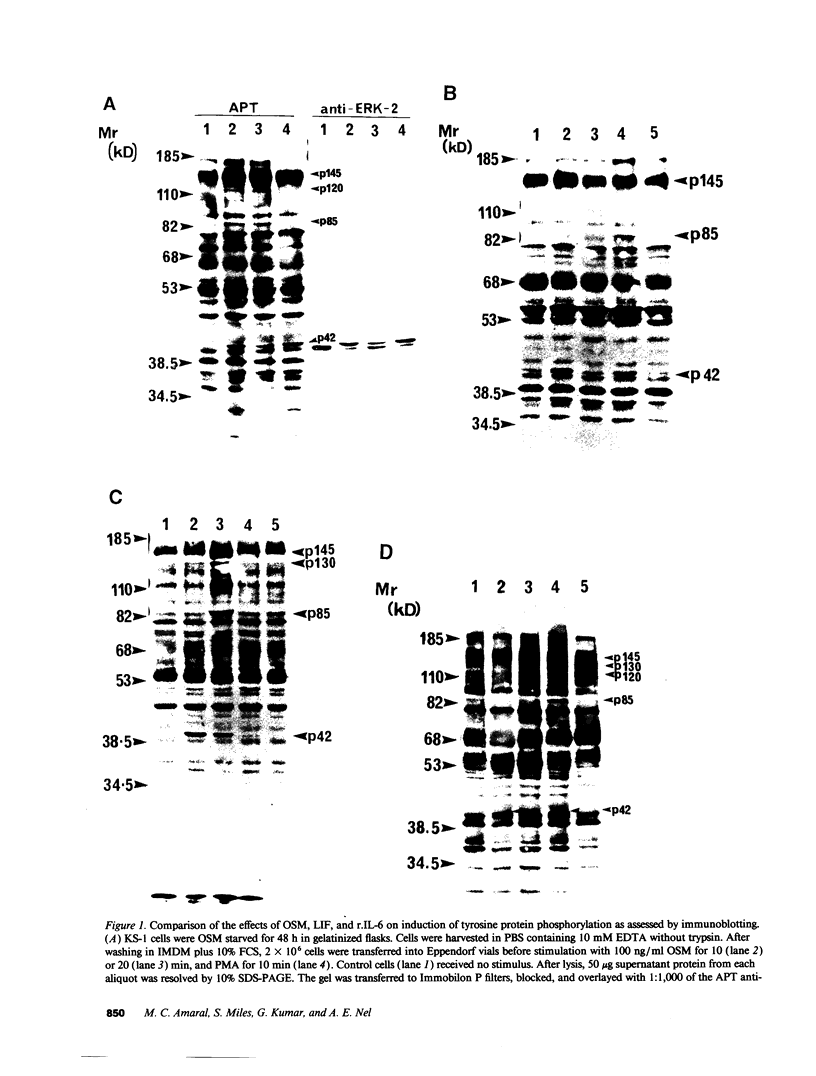

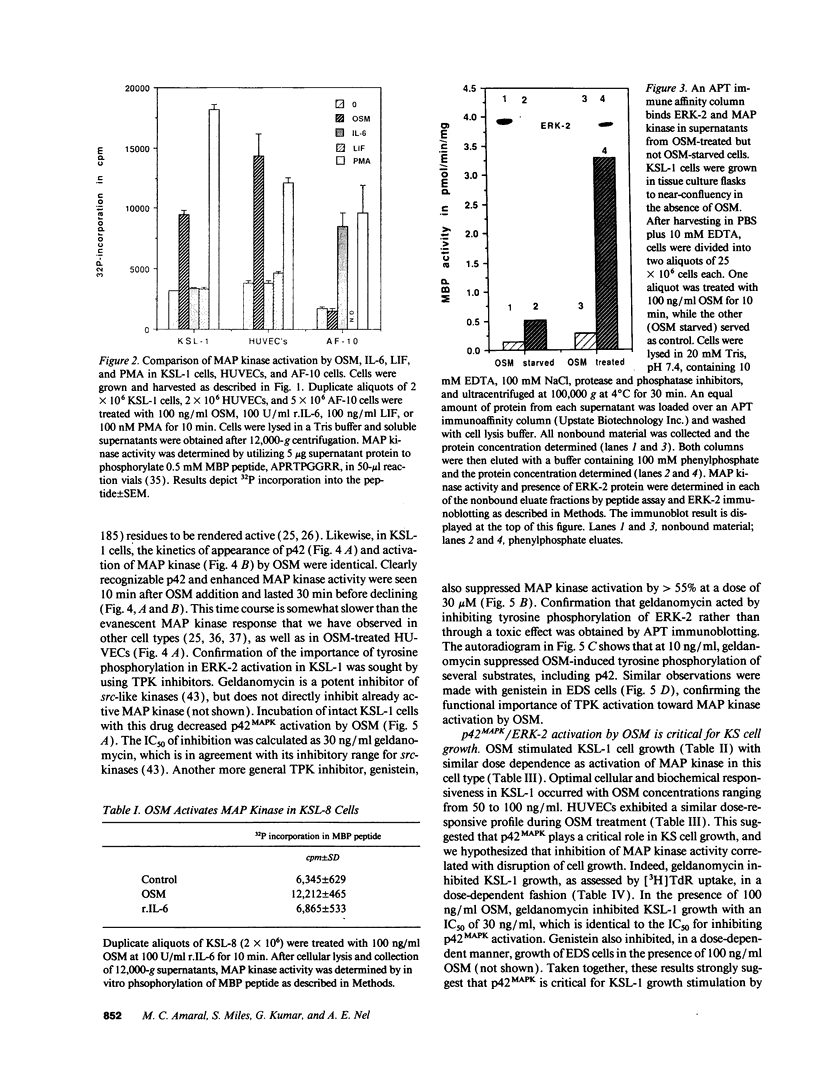
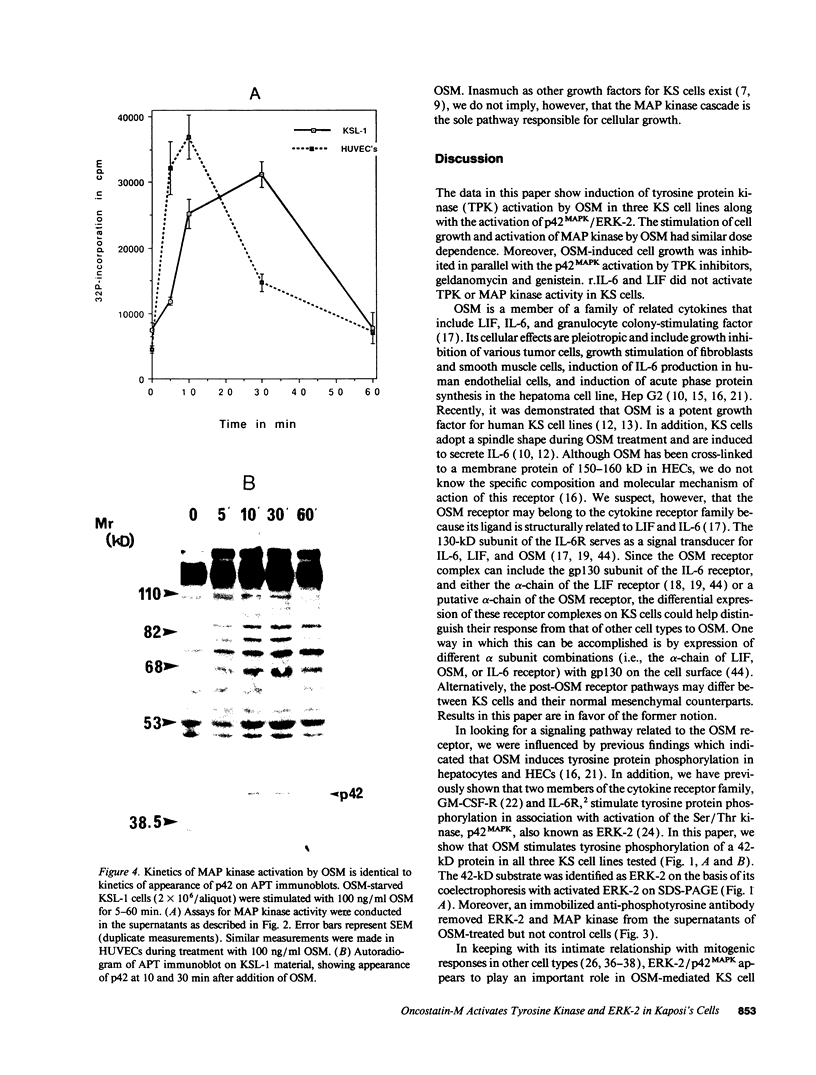



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Brown T. J., Rowe J. M., Liu J. W., Shoyab M. Regulation of IL-6 expression by oncostatin M. J Immunol. 1991 Oct 1;147(7):2175–2180. [PubMed] [Google Scholar]
- Casillas A., Hanekom C., Williams K., Katz R., Nel A. E. Stimulation of B-cells via the membrane immunoglobulin receptor or with phorbol myristate 13-acetate induces tyrosine phosphorylation and activation of a 42-kDa microtubule-associated protein-2 kinase. J Biol Chem. 1991 Oct 5;266(28):19088–19094. [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Corbeil J., Evans L. A., Vasak E., Cooper D. A., Penny R. Culture and properties of cells derived from Kaposi sarcoma. J Immunol. 1991 May 1;146(9):2972–2976. [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Gallo R. C. Pathogenesis of AIDS-associated Kaposi's sarcoma. Hematol Oncol Clin North Am. 1991 Apr;5(2):281–295. [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Salahuddin S. Z., Gallo R. C. AIDS-associated Kaposi's sarcoma: a molecular model for its pathogenesis. Cancer Cells. 1989 Nov;1(3):93–96. [PubMed] [Google Scholar]
- Ettehadieh E., Sanghera J. S., Pelech S. L., Hess-Bienz D., Watts J., Shastri N., Aebersold R. Tyrosyl phosphorylation and activation of MAP kinases by p56lck. Science. 1992 Feb 14;255(5046):853–855. doi: 10.1126/science.1311128. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., Comeau M. R., Friend D. J., Gimpel S. D., Thut C. J., McGourty J., Brasher K. K., King J. A., Gillis S., Mosley B. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992 Mar 13;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., Thut C. J., VandeBos T., Gimpel S. D., Delaney P. B., King J., Price V., Cosman D., Beckmann M. P. Leukemia inhibitory factor receptor is structurally related to the IL-6 signal transducer, gp130. EMBO J. 1991 Oct;10(10):2839–2848. doi: 10.1002/j.1460-2075.1991.tb07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove R. I., Mazzucco C. E., Radka S. F., Shoyab M., Kiener P. A. Oncostatin M up-regulates low density lipoprotein receptors in HepG2 cells by a novel mechanism. J Biol Chem. 1991 Sep 25;266(27):18194–18199. [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992 Oct 23;258(5082):593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- Kosako H., Gotoh Y., Matsuda S., Ishikawa M., Nishida E. Xenopus MAP kinase activator is a serine/threonine/tyrosine kinase activated by threonine phosphorylation. EMBO J. 1992 Aug;11(8):2903–2908. doi: 10.1002/j.1460-2075.1992.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Bolton-Hanson M., Horn D., Malik N., Kallestad J. C., Ochs V., Zarling J. M., Shoyab M. Identification and characterization of cellular receptors for the growth regulator, oncostatin M. J Biol Chem. 1989 Mar 15;264(8):4282–4289. [PubMed] [Google Scholar]
- Liu J., Modrell B., Aruffo A., Marken J. S., Taga T., Yasukawa K., Murakami M., Kishimoto T., Shoyab M. Interleukin-6 signal transducer gp130 mediates oncostatin M signaling. J Biol Chem. 1992 Aug 25;267(24):16763–16766. [PubMed] [Google Scholar]
- Miles S. A., Martínez-Maza O., Rezai A., Magpantay L., Kishimoto T., Nakamura S., Radka S. F., Linsley P. S. Oncostatin M as a potent mitogen for AIDS-Kaposi's sarcoma-derived cells. Science. 1992 Mar 13;255(5050):1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- Miles S. A., Rezai A. R., Salazar-González J. F., Vander Meyden M., Stevens R. H., Logan D. M., Mitsuyasu R. T., Taga T., Hirano T., Kishimoto T. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. C., DeVico A. L., Nakamura S., Copeland T. D., Chen Y., Patel A., O'Neil T., Oroszlan S., Gallo R. C., Sarngadharan M. G. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science. 1992 Mar 13;255(5050):1430–1432. doi: 10.1126/science.1542792. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Hultin L. Protein kinase C plays a role in the induction of tyrosine phosphorylation of lymphoid microtubule-associated protein-2 kinase. Evidence for a CD3-associated cascade that includes pp56lck and that is defective in HPB-ALL. J Immunol. 1991 Sep 15;147(6):1933–1939. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Rheeder A., Williams K., Pollack S., Katz R., Landreth G. E. Stimulation of MAP-2 kinase activity in T lymphocytes by anti-CD3 or anti-Ti monoclonal antibody is partially dependent on protein kinase C. J Immunol. 1990 Apr 1;144(7):2683–2689. [PubMed] [Google Scholar]
- Nel A. E., Ledbetter J. A., Williams K., Ho P., Akerley B., Franklin K., Katz R. Activation of MAP-2 kinase activity by the CD2 receptor in Jurkat T cells can be reversed by CD45 phosphatase. Immunology. 1991 Jun;73(2):129–133. [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Pollack S., Landreth G., Ledbetter J. A., Hultin L., Williams K., Katz R., Akerley B. CD-3-mediated activation of MAP-2 kinase can be modified by ligation of the CD4 receptor. Evidence for tyrosine phosphorylation during activation of this kinase. J Immunol. 1990 Aug 1;145(3):971–979. [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Raines M. A., Golde D. W., Daeipour M., Nel A. E. Granulocyte-macrophage colony-stimulating factor activates microtubule-associated protein 2 kinase in neutrophils via a tyrosine kinase-dependent pathway. Blood. 1992 Jun 15;79(12):3350–3354. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T. M., Bruce A. G. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8641–8645. doi: 10.1073/pnas.88.19.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford G. W., Schwarcz S. K., Lemp G. F., Barnhart J. L., Rauch K. J., Warner W. L., Piland T. H., Werdegar D. The epidemiology of AIDS-related Kaposi's sarcoma in San Francisco. J Infect Dis. 1989 Mar;159(3):569–572. doi: 10.1093/infdis/159.3.569. [DOI] [PubMed] [Google Scholar]
- Schieven G. L., Kallestad J. C., Brown T. J., Ledbetter J. A., Linsley P. S. Oncostatin M induces tyrosine phosphorylation in endothelial cells and activation of p62yes tyrosine kinase. J Immunol. 1992 Sep 1;149(5):1676–1682. [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Spandidos D. A., Kaloterakis A., Yiagnisis M., Varatsos A., Field J. K. Ras, C-myc and C-erbB-2 oncoprotein expression in non-AIDS Mediterranean Kaposi's sarcoma. Anticancer Res. 1990 Nov-Dec;10(6):1619–1625. [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Nakamura S., Shima T. B., Melchiori A., Martin G. R., Salahuddin S. Z., Gallo R. C., Albini A. Supernatants of acquired immunodeficiency syndrome-related Kaposi's sarcoma cells induce endothelial cell chemotaxis and invasiveness. Cancer Res. 1991 May 15;51(10):2670–2676. [PubMed] [Google Scholar]
- Uehara Y., Hori M., Takeuchi T., Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol Cell Biol. 1986 Jun;6(6):2198–2206. doi: 10.1128/mcb.6.6.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weich H. A., Salahuddin S. Z., Gill P., Nakamura S., Gallo R. C., Folkmann J. AIDS-associated Kaposi's sarcoma-derived cells in long-term culture express and synthesize smooth muscle alpha-actin. Am J Pathol. 1991 Dec;139(6):1251–1258. [PMC free article] [PubMed] [Google Scholar]
- Welham M. J., Duronio V., Sanghera J. S., Pelech S. L., Schrader J. W. Multiple hemopoietic growth factors stimulate activation of mitogen-activated protein kinase family members. J Immunol. 1992 Sep 1;149(5):1683–1693. [PubMed] [Google Scholar]
- Werner S., Hofschneider P. H., Heldin C. H., Ostman A., Roth W. K. Cultured Kaposi's sarcoma-derived cells express functional PDGF A-type and B-type receptors. Exp Cell Res. 1990 Mar;187(1):98–103. doi: 10.1016/0014-4827(90)90122-q. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Shoyab M., Marquardt H., Hanson M. B., Lioubin M. N., Todaro G. J. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9739–9743. doi: 10.1073/pnas.83.24.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]