Abstract
Infection associated with inert implants is complicated by bacterial biofilm formation that renders the infection antibiotic insensitive. The goal of this investigation was to synthesize and characterize a vancomycin (VAN)-modified bone allograft that could render the tissue inhospitable to bacterial colonization and the establishment of infection. We found that the numbers of primary amines, which could serve as anchors for chemical synthesis, increased with limited demineralization. Using these amines, we coupled two linkers and VAN to bone using Fmoc chemistry. By immunohistochemistry, VAN was abundant on the surface of the allograft; based on elution and measurement of bound antibody, this coupling yielded at least ∼26 ng VAN/mg bone. The coupled VAN appeared to be permanently bound to the allograft, as it showed no elution in a disk diffusion assay, and, importantly, resisted colonization by Staphylococcus aureus challenges. We suggest that this chimeric construct represents a new generation of antibiotic-modified allografts that provide antibacterial properties.
Introduction
Approximately 1.5 million bone and tissue allografts are distributed each year by American Association of Tissue Banks–accredited tissue banks in the United States.1 Grafting of this allograft has become a vital part of orthopedic surgery, and it is estimated that the use of bone allograft is required for more than 800,000 musculoskeletal procedures performed annually in the United States alone.2,3 Today bone graft is the second most often transplanted tissue3,4 exceeded only by blood. Implantation of synthetic or processed biomaterials into the human body is plagued by bacterial infection.5–8 Once bacteria adhere to a nonliving surface, they proliferate and, in a permissive environment, secrete a complex polymeric biofilm that protects the embedded bacteria from immune surveillance and greatly attenuates antibiotic effectiveness.9 Biofilm formation is particularly prevalent when bone allografts are utilized to promote tissue repair. These grafting materials are highly porous, noncellular, and avascular, and are thus inaccessible to immune surveillance, local cellular defense mechanisms,10 and systemic antibiotics. More than 11% of implanted bone grafts develop infection11–14 necessitating reoperations, removal of foreign material, debridement, and lengthy antibiotic treatment. To combat infection, grafts of synthetic materials that release high local concentrations of antibiotics from coatings or controlled release systems have been created.3,15,16 With bone in particular, direct adsorption of antibiotics to allograft is used as an elution system.17 These elution systems have met with varying degrees of success, and their use is limited by concerns over development of resistance, and ultimately re-establishment of infection as antibiotic elution wanes.
In previous reports, we have described and characterized the covalent bonding of antibiotics to metal surfaces using self-assembled monolayers of aminopropyltriethoxy silane.18,19 These covalently modified surfaces resist bacterial colonization in vitro,20,21 and appear to ameliorate the signs of infection in vivo.22 On the basis of these findings, we reasoned that it may be feasible to covalently link antibiotics directly to extracellular matrix (ECM) proteins of bone and other connective tissues to convert a passive allograft into a bioactive surface that resists bacterial colonization, while maintaining biocompatibility.
The antibiotic vancomycin (VAN) was chosen for these experiments based on both structural and activity criteria. First, the majority of deep orthopedic infections are caused by staphylococcus, and, in the face of an increasingly resistant array of microorganisms, VAN has become an important defense.23 Second, we have previously coupled VAN to metal surfaces via its carboxylic acid,18 making VAN a suitable candidate for this application. Third, others have reported derivatization of the VAN carboxylic acid with retention of activity.24 Fourth, VAN is active at the bacterial cell wall, ensuring that its target is readily accessible in its surface-bound state. Finally, in our hands, toxicity of VAN appears to only occur with relatively high antibiotic levels that would never be attained in these studies.21 For all of these reasons, VAN was chosen as a good candidate for coupling to allograft.
In this article, we describe the results of an investigation in which we have synthesized an antibiotic-modified bone allograft. We first asked whether sufficient primary amines were present in the bone matrix to allow for effective modification of the surface. We then chemically coupled two linkers and VAN to the allograft surface and asked if this coupling resulted in abundant VAN coverage of the allograft surface and how much VAN was associated with this modified surface. Finally, we tested the ability of the covalently modified surface to resist a bacterial challenge.
Materials and Methods
Experimental design
Morselized human bone (a mixture of porous cancellous and dense cortical bone morsels) and completely demineralized bone were evaluated for the presence of surface primary amines by fluorescamine staining. On the basis of these observations, we performed a surface demineralization of the allograft bone with 12.5% ethylenediaminetetraacetic acid (EDTA). As a function of time, calcium released from the bone was characterized by atomic absorption spectroscopy, and allograft surface amine coverage was detected with fluorescamine staining. In parallel, we assessed fully mineralized, demineralized, and partially demineralized bone (EDTA, 3 days) for surface amine coverage using fluorescamine staining; total amine content of these samples was determined using the colorimetric Ninhydrin assay. Using Fmoc chemistry, we then chemically coupled two linkers and the antibiotic VAN to the partially demineralized allograft (this demineralized allograft before any coupling is referred to as “control” allograft). Because the synthetic conditions could result in not only chemically coupled VAN, but also significant VAN adsorption, VAN-allograft was incubated in phosphate-buffered saline (PBS) to allow elution of any adsorbed VAN, and bone morsels were sampled daily for antibiotic elution by placing the samples on a uniform lawn of Staphylococcus aureus (S. aureus). Incubation in PBS was continued until no zones of inhibition were produced, indicating the conclusion of antibiotic elution. Using these allograft samples in which all adsorbed VAN had been eluted (VAN-bone), the retention of the covalently bound VAN on the allograft was detected by immunofluorescence, with digital imaging by confocal microscopy. In parallel, release of the fluorescent antibodies from the VAN-bone surfaces was used to measure total VAN amounts bonded to the VAN-bone surface. Finally, we tested whether VAN-alograft would resist colonization by S. aureus, with viable, surface-adhered bacteria detected by fluorescent staining followed by digital imaging. In parallel, using both VAN and control bone, adherent bacteria were suspended by sonication and measured after serial dilution and plating.
Allograft preparation
Cancellous/cortical human bone granules 0.5–2 mm (Musculoskeletal Transplant Foundation) were washed and sonicated with dH2O until washings were clear. Where indicated, samples were partially demineralized with 12.5% EDTA (pH 7) at room temperature (RT) for 3 days with shaking, and daily replacement of the EDTA. Before synthesis, all samples were washed with dH2O and sonicated twice for 30 min in dimethylformamide (DMF; Acros Organics, Fair Lawn, NJ).
Assessment of primary amines
Morselized bone was incubated with 12.5% EDTA (pH 7) at RT for 10 days with shaking; eluent containing dissolved calcium was replaced daily with fresh EDTA. After its dilution with 0.5 N HCl and 0.1% LaCl3, this eluent was assessed by atomic adsorption spectroscopy to determine the Ca2+ content. To determine surface amine distribution, these morselized bone samples were washed thrice with acetone and incubated in 1 mg/mL of fluorescamine in acetone for 40 min in the dark. After washing thrice with acetone to remove free dye, bound fluorescence was observed by confocal laser scanning microscopy. To measure total amounts of primary amines, 10 mg dry weight of morselized allograft was boiled in a water bath for 15 min in 100 μL 3.5 m SnCl2,/100 μL 1M Na3C6H5O7·2H2O pH 5/1 ml 4% Ninhydrin in ethanol and 800 μL dH2O.18 The absorbance of the supernatant was measured and the molar concentration of primary amines calculated (ɛ570 = 1.5 × 104 [M-cm]− 1).
Allograft modification
Washed allograft was coupled with 10 mg/mL Fmoc-[2-(2-amino-ethoxy)-ethoxy]–acetic acid in DMF/diisopropyle-thylamine (100:1) in the presence of O-(7-azabenzo-triazole-1-yl)-1,1,3,3-tetramethyluronium hexa-fluorophosphate (HATU), 2 h, room temperature, followed by washing 10 times with DMF. The linker was deprotected by treatment with 20% piperidine in DMF 30 min and washed 10 times with DMF, and a second linker was coupled and deprotected as above. After the final Fmoc deprotection, samples were coupled with 10 mg/mL clinical-grade VAN (American Pharmaceutical Partners, Schaumburg, IL) in DMF/diisopropymine, in the presence of HATU, for 12–16 h. The modified bone was washed extensively with DMF and dH2O.
Antibiotic elution
VAN adsorption to control or VAN-allograft was measured by elution in dH2O with shaking. From these eluting batches, a bone granule was placed daily onto an agar plate that had been preseeded with uniform bacterial lawns of S. aureus. Plates were incubated at 37°C overnight and observed for zones of inhibition radiating from the granules. Incubation of allograft in dH2O was continued until there were no zones of inhibition, indicating cessation of elution of adsorbed VAN.
Immunohistochemical detection and quantization of VAN
Control or VAN-allograft was washed thrice with PBS, blocked with 10% fetal bovine serum in PBS (blocking buffer) for 1 h, and incubated with rabbit anti-VAN IgG (1:500; US Biologicals, Swampscott, MA) in blocking buffer at 4°C for 12 h. Samples were washed thrice with PBS and incubated with an AlexaFluor 488-goat anti-rabbit IgG secondary antibody (1:1000; Molecular Probes/Invitrogen, Carlsbad, CA) at RT for 1 h, and washed thrice with PBS. These fluorescently stained samples were observed by epifluorescence, or 30 mg of these control and VAN-allograft samples was incubated with 1 ml of 7 M urea with slow rocking at 4°C overnight. The eluted fluorescent antibody was measured (λEx = 494 nm; λEm = 520 nm, TECAN plate reader), and VAN concentrations were calculated by comparison to a standard curve of known concentrations of the secondary antibody in 7 M urea.
Bacterial culture and challenge
Ten milligrams dry weight of control or of VAN-allograft was sterilized with 70% ethanol for 15 min, and washed thrice with PBS and thrice with trypticase soy broth (TSB). S. aureus (Xen36 derived from ATCC 49525; Caliper Life Science, Hopkinton, MA) were cultured in TSB at 250 rpm and 37°C for 12–14 h (overnight culture). Using a 0.5 McFarland standard (a turbidity measure in which A600 = 0.10 for ∼1 × 108 cfu/mL), 1 × 104 cfu/mL bacteria were incubated with the sterilized samples in TSB at 37°C under static conditions. Upon harvesting, samples were washed thrice with PBS to remove nonadherent bacteria, and either (1) stained with the Live/Dead BacLight™ Kit (based on the differential membrane permeability of Syto9 and propidium iodide; Invitrogen, Carlsbad, CA; 20 min, RT) and fluorescent bacteria were observed with confocal laser microscopy (Olympus Fluoview 300, Center Valley, PA), or (2) washed three more times and sonicated in 0.3% Tween-80 for 5 min to suspend adherent bacteria, which were then serially diluted, and suspended bacteria were plated on 3M® Petrifilms (3M Microbiology, St. Paul, MN). Petrifilms were scanned and colonies counted using a macro in Adobe Photoshop CS3.
Statistical analysis
All experiments were performed independently at least thrice. Data are presented as means ± standard errors. All statistical analyses were performed on normal equally variant data, unless indicated otherwise in the legend, using a one- or two-way analysis of variance with a Tukey multiple comparison procedure. An α of 0.05 was considered significant.
Results
Surface amine availability is increased with demineralization
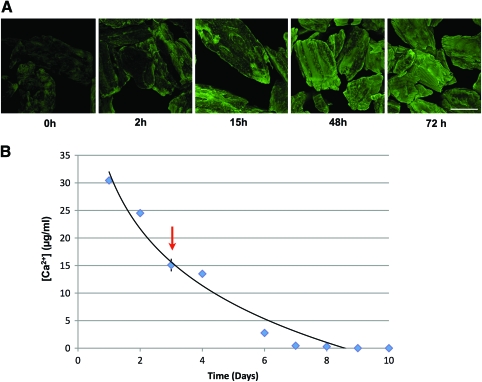
We first evaluated morselized bone for the presence of primary amines that could be used as anchors for attachment of VAN. Fully mineralized bone was stained with fluorescamine, which binds to primary amines. Staining appeared patchy with areas of intense staining adjacent to areas showing little to no fluorescence, indicating that limited surface amines were available for chemical coupling (0 h, Fig. 1A). With as little as 2 h incubation in 12.5% EDTA, fluorescamine staining increased. By 15 h incubation in EDTA, fluorescamine staining was largely punctate and interspersed with large areas of intense staining. These intensely fluorescent areas increased in size, until by 72 h, fluorescence was uniformly intense over the surface of the morsels. To determine the extent of demineralization during these treatments, Ca2+ content of the EDTA solution over the demineralization course of 10 days was monitored by atomic adsorption spectroscopy (Fig. 1B). The Ca2+ concentration of the EDTA solution was highest after the first day of demineralization, reaching ∼30 μg Ca2+/mL. Ca2+ concentrations then decreased gradually until they were no longer detectable by day 10. Importantly, the Ca2+ content extracted from the bone during the first 3 days of incubation (arrow) is ∼60% of the total Ca2+ content of the bone.
FIG. 1.
Surface demineralization of morselized bone. (A) Primary amines were further exposed by demineralization for 0–72 h, labeled with fluorescamine, and observed by confocal microscopy. Note that the fluorescent signal becomes more intense as a function of time, implying increased primary amine availability. Scale bar = 400 μm. (B) Calcium content during demineralization. [Ca2+] in the EDTA bathing solution was determined by atomic adsorption spectroscopy. The graph shows the calculated value of [Ca2+] as a function of time of demineralization. Note that even after 3 days of demineralization (arrow), abundant Ca2+ still remains in the bone core. EDTA, ethylenediaminetetraacetic acid. Color images available online at www.liebertonline.com/ten.
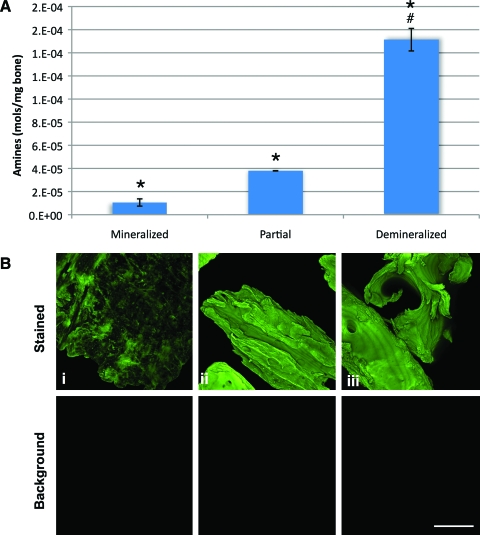
We then directly measured the effect of demineralization on amine availability using the Ninhydrin assay, which forms a colored adduct in the presence of primary amines.18 The untreated, mineralized bone contained ∼1 × 10−5 mol of primary amine/mg of morselized bone; demineralization for 3 days increased the amount of available amines ∼4-fold (∼3.8 × 10−5 mol/mg bone). Demineralized bone had the greatest number of available primary amines, at ∼1.8 × 10−4 mol/mg bone (Fig. 2A). When parallel samples were stained with fluorescamine (Fig. 2B), mineralized bone had the patchy staining pattern noted in Figure 1. Bone demineralized for 3 days had a fluorescamine intensity similar to that of fully demineralized bone (Fig. 2B, ii vs. iii). On the basis of our data from Figure 1, this staining pattern indicated that partial demineralization predominantly released the readily available mineral, that is, surface mineral. Thus, this 3-day treatment (referred to as “partially demineralized bone”) was used as a starting point for all further modifications.
FIG. 2.
Amine content and coverage of bone. (A) The primary amine content of bone after 0, 3, or 10 days of demineralization, as determined using the Ninhydrin assay. Fully mineralized bone yielded the lowest concentration of amines. Bone that was treated with 12.5% EDTA for 3 days (partial) had significantly increased levels of primary amine availability but was still significantly less than that of fully demineralized bone (*p < 0.005 from mineralized, #p < 0.005 from partial). (B) Primary amines on the bone surface were observed by fluorescamine staining. Shown are (i) fully mineralized bone, (ii) partially demineralized bone, and (iii) fully demineralized bone. Scale bar = 400 μm. Color images available online at www.liebertonline.com/ten.
VAN can be bonded to bone amines
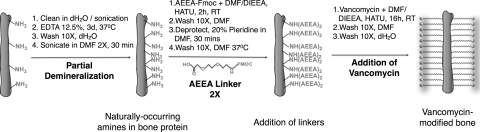
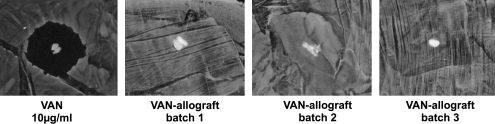
Using partially demineralized bone (which is used as control bone in all subsequent experiments), two 2-(2-amino-ethoxy)-ethoxy]-acetic acid linkers were sequentially coupled to the allograft, followed by attachment of VAN via its carboxylic acid group to the linkered surface (Scheme, Fig. 3). During the synthetic process, allograft is exposed to high concentrations of VAN, allowing VAN adsorption as well as covalent bonding. Therefore, we monitored VAN elution from VAN-allograft by placing sample morsels on a uniform S. aureus lawn daily and comparing the resulting zones of inhibition with those from 10 μg/mL VAN adsorbed to control bone (Fig. 4, VAN). VAN elution from this control bone causes a clear zone of inhibition centered on the bone morsel where S. aureus proliferation is suppressed due to high antibiotic concentrations. When VAN-allograft is tested for elution, small amounts of the antibiotic diffuse from the morsels; as its concentration drops, zones of inhibition become progressively smaller. After 3–5 days (shown for three different batches of VAN-allograft), zones of inhibition are no longer apparent, indicating that no adsorbed VAN remains in the VAN-allograft. This elution monitoring was used for every batch of VAN-allograft to ensure that only covalently bonded VAN-allograft was tested.
FIG. 3.
Synthetic scheme. Morselized allogenic bone was cleaned by sonication in dH2O and partially demineralized with 12.5% EDTA for 3 days. After further washing, it was sonicated for 30 min in DMF. It was then coupled twice with Fmoc-AEEA in DMF/DIEA, washed, and deprotected by treatment with 20% piperidine in DMF. After the final Fmoc deprotection, samples were coupled with 10 mg/mL clinical-grade VAN in DMF/DIEA in the presence of HATU and washed extensively with DMF and dH2O. VAN, vancomycin; DMF, dimethylformamide; Fmoc-AEEA, Fmoc-[2-(2-amino-ethoxy)-ethoxy]-acetic acid; DIEA, diisopropylethylamine.
FIG. 4.
Elution of adsorbed antibiotic from newly synthesized VAN-allograft. A morsel of bone that had had VAN adsorbed to it (VAN, 10 μg/mL) or a morsel of VAN-allograft after 3–5 day elution was tested for elution of VAN on a uniform Staphylococcus aureus lawn. The VAN-impregnated allograft eluted sufficient VAN to cause growth inhibition around the bone morsel. In contrast, the three different batches of VAN-allograft, after a 3–5 day elution, contained insufficient adsorbed VAN to inhibit bacterial growth resulting in no detectable clear zone around the morsels.
VAN has a uniform distribution on VAN-allograft
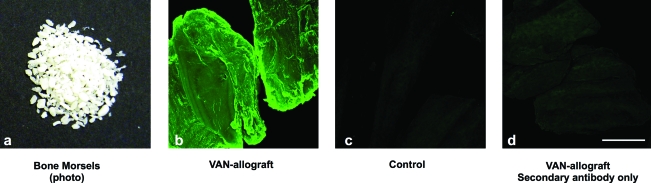
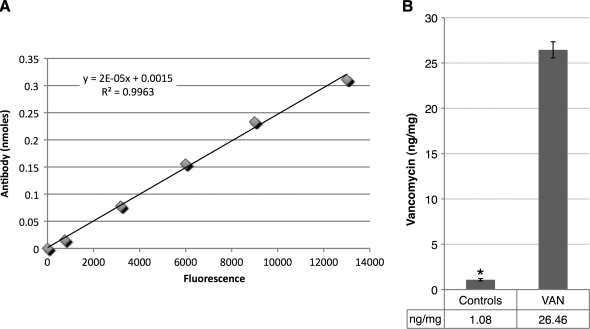
After the 3–5 days of elution, we evaluated the VAN allograft (shown in Fig. 5a) for VAN distribution using anti-VAN antibodies. By immunofluorescence, intense VAN staining was apparent on the VAN-allograft (Fig. 5b), whereas control unmodified bone showed only a diffuse background fluorescence (Fig. 5c). Staining of the VAN-allograft with secondary antibody alone showed no appreciable signal, confirming the specificity of the observed staining (Fig. 5d). To approximate VAN amounts bonded to the bone matrix, anti-VAN antibodies were dissociated from both control and VAN-allograft by treatment with 7 M urea. A secondary antibody standard curve (Fig. 6A) was generated; after conversion of released amounts, control bone released antibody equivalent to 1.08 ng VAN/mg bone (background), whereas ∼26 ng VAN/mg bone was calculated for VAN-allograft (Fig. 6B).
FIG. 5.
Visual assessment of VAN attachment to bone. (a) Morselized bone used for the synthesis of VAN-allograft. (b) After incubation with anti-VAN antibody, VAN-allograft displayed a uniform, intense fluorescence covering the whole surface of the bone, indicating successful modification of the graft. (c) Control allograft stained with the anti-VAN antibody exhibited only minimal background fluorescence representing the natural fluorescence of bone. (d) As a negative control, VAN-allograft was incubated with secondary antibody alone. Color images available online at www.liebertonline.com/ten.
FIG. 6.
Measurement of immobilized VAN on VAN-allograft. (A) Standard curve for fluorescence of AlexaFluor488 secondary antibody (0–0.2 μg/mL) in the 7M urea extraction buffer. Note that the R2 value is close to 1. (B) Graphical representation of amounts of antibody recovered from control or VAN-allograft. Approximately 26 ng of immobilized antibiotic per mg of morselized bone was calculated from the standard curve. Controls showed a low VAN level due to limitations of the assay. *p < 0.005.
VAN-allograft resists colonization by S. aureus
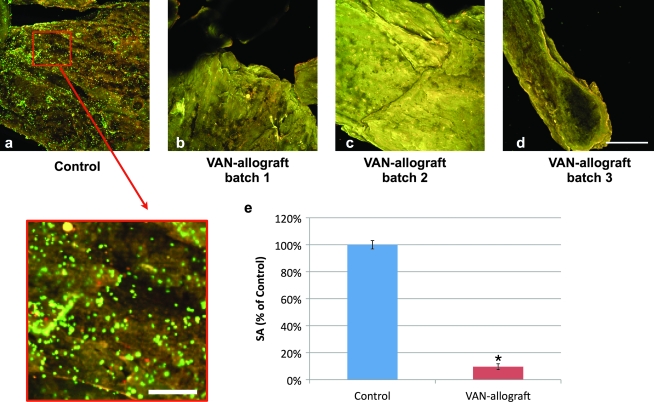
Finally, we tested the ability of VAN-allograft to resist colonization with S. aureus. After a 6 h S. aureus challenge (Ci = 104 cfu), control bone was abundantly colonized, with the surface of the morsels occupied by the fluorescently stained S. aureus (Fig. 7a). Bacteria were organized in microcolonies (individual bright green dots) and in continuous clusters that are indicative of biofilm formation (Fig. 7, inset). VAN-allograft from three separate syntheses was able to resist colonization and appeared free of bacterial attachment (Fig. 7b–d). In addition, adherent bacteria were recovered and counted; bacterial loads were reduced by up to 90% on VAN-allograft as compared to controls (Fig. 7e).
FIG. 7.
VAN-allograft resists S. aureus colonization. Control or VAN-allograft were challenged with S. aureus (Ci = 104 cfu) for 6 h and adherent bacteria were observed using the Live/Dead assay. (a) Abundant viable (green) and some dead (yellow) bacteria were detected on control samples that uniformly covered the bone surface. (a, insert) Bacteria are also seen organizing in clusters producing areas of Live/Dead (Syto9 and Propidium Iodide) stain uptake that may mark the initiation of biofilm formation. (b–d) In sharp contrast, few, if any, dispersed bacteria were present on VAN-allograft from three different synthetic batches. VAN-allograft consistently appeared devoid of bacterial colonization and only exhibited a uniform yellow appearance produced from the natural fluorescence of the bone surface itself. (e) Adherent bacteria were released from the surface of bone by sonication and quantified by serial dilution and plating. VAN-allograft was able to resist colonization and significantly reduced the bacterial load on bone grafts by > 90%. *p < 0.05. Scale bars: (a–d) 200 μm (a, insert) 50 μm.
Discussion
Allogenic grafts are harvested from human cadavers and extensively used in clinical practice to fill bony defects encountered in orthopaedics, dentistry, and medicine. Infection remains one of the most devastating complications associated with bone graft impaction. The goal of this investigation was to explore the feasibility of covalently bonding VAN to morselized allograft bone to protect the allograft from bacterial colonization. The first step was to ensure that the allograft bone surface was suitable for covalent modification by presentation of accessible anchor points, that is, primary amines. We determined that the density of the surface amines could be optimized by partial demineralization. We then described a synthetic scheme that allowed attachment of the antibiotic VAN though two flexible linkers. That only VAN bonded to bone was evaluated was ensured by monitoring elution of any adsorbed VAN into aqueous solutions. By immunostaining, the synthesis promoted uniform tethering of the antibiotic to the allograft, with antibiotic release indicating that the yield was ∼25 ng/mg of morselized bone. This determination was limited in that it assumes a 1:1:1 correspondence of VAN:primary:secondary antibodies. We recognize that given the size of VAN (∼1.7 kDa), it is unlikely that an α-VAN antibody (∼150 kDa) will be able to bind to each VAN. We used this determination as a minimum concentration of VAN on the surface, rather than an absolute amount. The VAN maintained its antimicrobial properties and remained active against gram-positive organisms effectively inhibiting S. aureus colonization. Again, the pictures that we present are limited as we clearly recovered colonizing bacteria from the VAN-allograft. The clear reduction in bacterial load, based on our experience from other systems, is sufficient to cause a real difference in the outcome of infection.
In an earlier study18 we used silane chemistry to first aminopropylate oxidized Ti to display a primary amine, followed by bonding of two linkers and antibiotics to the titanium surface via coupling to this amine. Thus, in evaluating the suitability of bone for a similar scheme, we first needed to determine the availability of surface amines. Specifically, decellularized bone is comprised of an organic, predominantly collagenous matrix that is impregnated with inorganic, calcium-rich mineral.25 This ECM is an attractive option for attachment since the collagenous component should be rich in amino acids that bear side chains with primary amines. Nevertheless, recent modeling of collagen suggests that the majority of primary amines may be clustered in highly charged pockets in the gap regions of collagen fibrils. These same sites are thought to act as nucleation sites for initiation of mineral deposition.26 Thus, we hypothesized that access to this region would be critical for modifying the collagenous ECM. Indeed, demineralization and by implication, exposure of these primary amine-containing pockets significantly increased amine availability, as detected by the Ninhydrin assay and fluorescamine staining.
Our limited partial demineralization resulted in apparently uniform amine exposure on the surface of the allograft with fluorescence intensity akin to that of fully demineralized allograft. Nevertheless, the total amine content of this partially demineralized bone was only moderately greater than fully mineralized bone and significantly lower than fully demineralized bone. Thus, it appeared that the majority of amines must still be entrenched in the bone mineral, and that we had performed a surface demineralization. Importantly, when we measured released Ca2+ during this partial demineralization, it appeared that demineralization at the early time points was very effective at exposing the surface amines, while preserving much of the mineral in the core of the bone, thereby preserving most of its structural and mechanical stability. It is worth noting that this result was obtained with a mixture of cortical and cancellous bone and that the appearances were sufficiently similar as to allow no discrimination based on microscopy.
We, of course, have wondered which proteins supply the primary amines for VAN bonding. We hypothesize that collagen type I will be a major site for VAN bonding, both because of its abundance in the bone matrix and because of the effect of demineralization on exposure of primary amines. Use of this primary amine allows formation of a peptide bond between it and the carboxylic acid of VAN. Importantly, this peptide bond should not affect VAN activity. From a structural perspective, this carboxylic acid falls outside of the region mapped for peptidoglycan binding and thus should not affect the active site of the antibiotic. The microbiological and immunohistochemical studies lent strong support to this contention, indicating that the coupling chemistry maintained the full spectrum of VAN activity.
Chemical modification of this construct with VAN was performed on multiple independent batches of morselized bone to ensure reproducibility not only in successful attachment of the antibiotic but also in its biocidal activity. Before evaluation of the VAN-allograft, it was incubated in aqueous medium until no zones of inhibition (indicating antibiotic elution) were produced on uniform bacterial lawns. Immobilization instead of impregnation of antibiotic onto the grafts has several implications. First, the antibiotic does not dissociate from the graft and therefore does not get depleted. Second, it is expected to stay on the surface of the bone, providing long-term protection that may withstand multiple challenges. Finally, no systemic toxicity is expected since the antibiotic is limited to the surface of the graft where it inhibits the attachment of bacteria. Even under conditions where the bone matrix is resorbed, antibiotic release would be small and therefore not cause toxicity.
The predicted long-term protection of the chemically bonded VAN-allograft was borne out in studies examining the ability of the VAN-allograft to withstand a bacterial challenge. VAN-allograft resisted bacterial colonization and, when compared to untreated controls, reproducibly reduced the bacterial load by >90%. The coagulase-negative Staphylococci that were used for this study represent the main culprit in graft-associated infections and account for 36% to 38% of all allograft infections.11,12 Further, these bacteria are notoriously efficient biofilm producers making eradication of established infections nearly impossible.27 By inhibiting the initial step of attachment, we hypothesize that biofilm formation can be prevented.
Finally, it should be noted that while the current study is limited to allograft bone, we anticipate that the antibiotic-tethering procedure will be applicable to other decellularized grafts, such as vascular grafts. Since the tethering relies on primary amine availability in the collagenous matrix, it is conceivable that the same technology can be extended to prosthetic heart valves,28,29 decellularized hearts,30 and other native nonviable tissues. Importantly, the methods described in this report could be used to tether other antibiotics, chemotherapeutics, and chemotactic, osteogenic, angiogenic, and antithrombotic factors to the graft.31 Thus, covalent modification of native tissues represents an important advance in enhancing functionality and preventing the devastating complications of uncontrolled infections.
Acknowledgments
The authors thank the Musculoskeletal Transplant Foundation for providing samples for this work as well as for their generous support of these studies. The authors thank the NIH (Grants DE-13319, DE-10875, and AR-051303) and the Department of Defense (Grant DAMD17-03-1-0713) for funding this study. Results presented are not the statement or policy of the funding agencies. The authors would also like to thank Gerald Harrison (University of Pennsylvania) for help with atomic absorption spectroscopy.
Ethics Board Review Statement
No animals or human subjects.
Disclosure Statement
The authors have filed an invention disclosure with Thomas Jefferson University.
References
- 1.Centers for Disease Control and Prevention. www.cdc.gov. 2010. www.cdc.gov
- 2.Enneking W.F. Mindell E.R. Burchardt H. Tomford W. Allograft safety and ethical considerations: editorial comment. Clin Orthop Rel Res. 2005;435:2. [Google Scholar]
- 3.Lewandrowski K. Gresser J. Wise D. Trantol D. Bioresorbable bone graft substitutes of different osteoconductivities: a histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials. 2000;21:757. doi: 10.1016/s0142-9612(99)00179-9. [DOI] [PubMed] [Google Scholar]
- 4.Boyce T. Edwards J. Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin N Am. 1999;30:571. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 5.Souli M. Giamarellou H. Effects of slime produced by clinical isolates of coagulase-negative staphylococci on activities of various antimicrobial agents. Antimicrob Agents Chemother. 1998;42:939. doi: 10.1128/aac.42.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttaro M. Comba F. Piccaluga F. Vancomycin-supplemented cancellous bone allografts in hip revision surgery. Clin Orthop Relat Res. 2007;461:74. doi: 10.1097/BLO.0b013e318073c290. [DOI] [PubMed] [Google Scholar]
- 7.Keidar Z. Engel A. Hoffman A. Israel O. Nitecki S. Prosthetic vascular graft infection: The role of 18F-FDG PET/CT. J Nucl Med. 2007;48:1230. doi: 10.2967/jnumed.107.040253. [DOI] [PubMed] [Google Scholar]
- 8.Chang J. Calligaro K. Ryan S. Runyan D. Dougherty M. Stern J. Risk factors associated with infection of lower extremity revascularization: analysis of 365 procedures performed at a teaching hospital. Ann Vasc Surg. 2003;17:91. doi: 10.1007/s10016-001-0337-8. [DOI] [PubMed] [Google Scholar]
- 9.Costerton J.W. Stewart P.S. Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 10.Witso E. Persen L. Benum P. Bergh K. Cortical allograft as a vehicle for antibiotic delivery. Acta Orthop. 2005;76:481. doi: 10.1080/17453670510041457. [DOI] [PubMed] [Google Scholar]
- 11.Lord C. Gebhardt M. Tomford W. Mankin H. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369. [PubMed] [Google Scholar]
- 12.Tomford W. Thongphasuk J. Mankin H. Ferraro M. Frozen musculoskeletal allografts. A study of the clinical incidence and causes of infection associated with their use. J Bone Joint Surg Am. 1990;72:1137. [PubMed] [Google Scholar]
- 13.Aro H. Aho A. Clinical use of bone allografts. Ann Med. 1993;25:403. doi: 10.3109/07853899309147303. [DOI] [PubMed] [Google Scholar]
- 14.Gross A. Hutchison C. Alexeeff M. Mahomed N. Leitch K. Morsi E. Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip. Clin Orthop Relat Res. 1995;319:151. [PubMed] [Google Scholar]
- 15.Bauer T. Bone graft substitutes. Skelet Radiol. 2007;36:1105. doi: 10.1007/s00256-007-0377-4. [DOI] [PubMed] [Google Scholar]
- 16.Beardmore A. Effectiveness of local antibiotic delivery with an osteoinductive and osteoconductive bone-graft substitute. J Bone Joint Surg Br. 2005;87:107. doi: 10.2106/JBJS.C.01670. [DOI] [PubMed] [Google Scholar]
- 17.Winkler H. Kaudela K. Stoiber A. Menschik F. Bone grafts impregnated with antibiotics as a tool for treating infected implants in orthopedic surgery—one stage revision results. Cell Tissue Bank. 2006;7:319. doi: 10.1007/s10561-006-9010-3. [DOI] [PubMed] [Google Scholar]
- 18.Jose B. Antoci V., Jr. Zeiger A.R. Wickstrom E. Hickok N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem Biol. 2005;12:1041. doi: 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Edupuganti O.P. Antoci V., Jr. King S.B. Jose B. Adams C.S. Parvizi J. Shapiro I.M. Zeiger A.R. Hickok N.J. Wickstrom E. Covalent bonding of vancomycin to Ti6Al4V alloy pins provides long-term inhibition of Staphylococcus aureus colonization. Bioorg Med Chem Lett. 2007;17:2692. doi: 10.1016/j.bmcl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Antoci V., Jr. Adams C.S. Parvizi J. Davidson H.M. Composto R.J. Freeman T.A. Wickstrom E. Ducheyne P. Jungkind D. Shapiro I.M. Hickok N.J. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29:4684. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoci V., Jr. King S.B. Jose B. Parvizi J. Zeiger A.R. Wickstrom E. Freeman T.A. Composto R.J. Ducheyne P. Shapiro I.M. Hickok N.J. Adams C.S. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 22.Antoci V., Jr. Adams C.S. Hickok N.J. Shapiro I.M. Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007;461:88. doi: 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- 23.Peersman G. Laskin R. Davis J. Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15. [PubMed] [Google Scholar]
- 24.Loll P.J. Axelsen P.H. The structural biology of molecular recognition by vancomycin. Ann Rev Biophys Biomol Struc. 2000;29:265. doi: 10.1146/annurev.biophys.29.1.265. [DOI] [PubMed] [Google Scholar]
- 25.Anderson H. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995;314:266. [PubMed] [Google Scholar]
- 26.Landis W. Silver F. Mineral deposition in the extracellular matrices of vertebrate tissues: identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs. 2009;189:20. doi: 10.1159/000151454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fux C. Costerton J. Stewart P. Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Schopka S. Schmid F. Hirt S. Birnbaum D. Schmid C. Lehle K. Recellularization of biological heart valves with human vascular cells: in vitro hemocompatibility assessment. J Biomed Mater Res Part B Appl Biomater. 2009;88:130. doi: 10.1002/jbm.b.31159. [DOI] [PubMed] [Google Scholar]
- 29.Rieder E. Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792. doi: 10.1161/CIRCULATIONAHA.104.473629. [DOI] [PubMed] [Google Scholar]
- 30.Ott H. Matthiesen T. Goh S. Black L. Kren S. Netoff T. Taylor D. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 31.Ye X. Zhao Q. Sun X. Li H. Enhancement of mesenchymal stem cell attachment to decellularized porcine aortic valve scaffold by in vitro coating with antibody against CD90: a preliminary study on antibody-modified tissue-engineered heart valve. Tissue Eng A. 2008;15:1. doi: 10.1089/ten.tea.2008.0001. [DOI] [PubMed] [Google Scholar]