Figure 1.
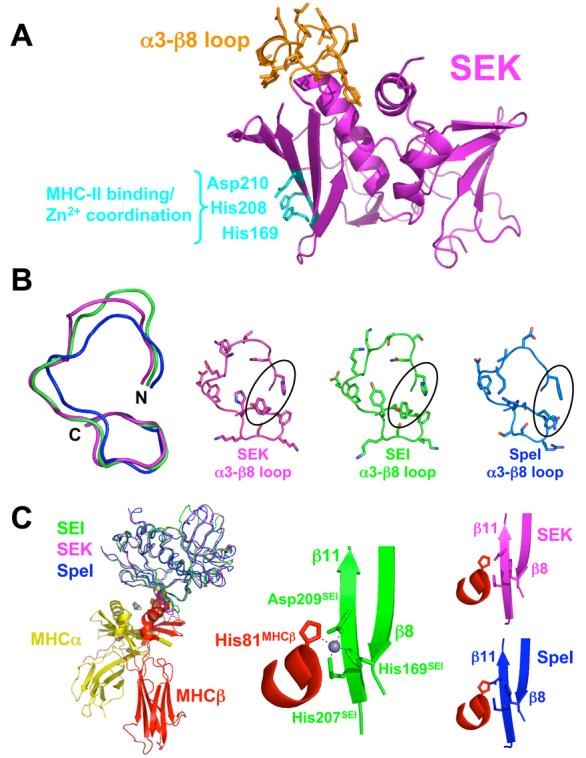
Structural similarity of SEK and other Group V bacterial superantigens. (A) Structure of SEK in the unbound state. The α3-β8 loop is in orange and the residues responsible for Zn2+ coordination and MHC class II binding are in cyan. (B) Structural comparison of the α3-β8 loop domains of SEK, SEI and SpeI. Superposition of the main chains of the α3-β8 loops (left panel). Molecular detail of side chain positions in the α3-β8 loops (right panels). SEK, SEI and SpeI are in magenta, green and blue, respectively. The residues in SEK that interact with TCR, His142 and Tyr158, as well as corresponding residues in SEI and SpeI are encircled. (C) Structural comparison of the MHC binding site of SEI and the putative MHC binding sites of SEK and SpeI. Superposition of SEK and SpeI with SEI from the SEI-MHC class II crystal structure22 (left panel). Close-up view of the Zn2+ coordination between SEI residues His169, His207 and Asp209 and the MHC β subunit residue His81 (middle panel). Close-up views of the putative SAG-MHC interface formed by the superposed SEK and SpeI structures (right panels). The MHC α subunit is in yellow, MHC β subunit is in red, zinc ion in gray, and the SAG colors are as in panel B.
