Figure 2.
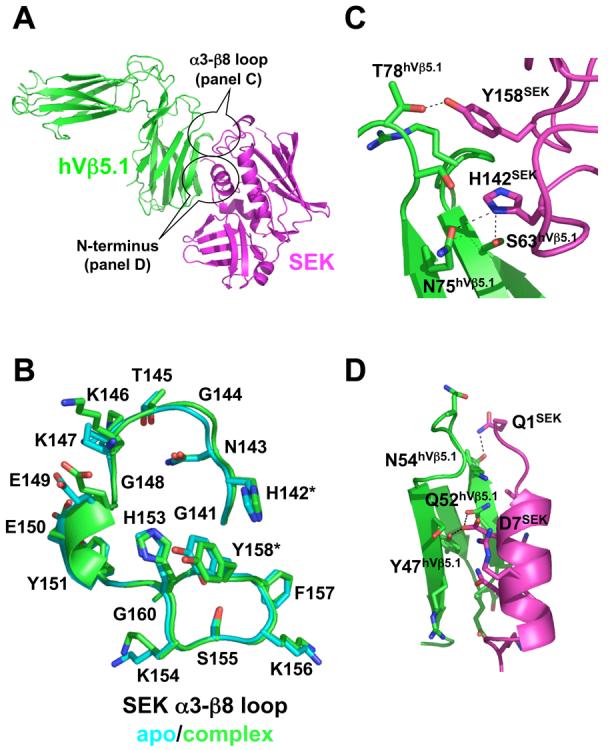
Structure of the SEK-hVβ5.1 complex. (A) Cartoon representation of the interaction between SEK and hVβ5.1. SEK is in magenta, hVβ5.1 is in green. The two regions of SEK, the α3-β8 loop and the N-terminus, that contact the TCR are encircled. (B) Comparison of SEK α3-β8 loop structures in the unbound (cyan) and hVβ5.1-bound (green) crystal structures. The residues that make intermolecular contacts with hVβ5.1, His142 and Tyr158, are demarcated by asterisks. (C) Close-up view of the interface formed by the α3-β8 loop of SEK and the FR3 and FR4 loops of hVβ5.1. (D) Close-up view of the interface formed by the N-terminus of SEK and the CDR2 loop of hVβ5.1. In both panels C and D, the side chains of only those residues that make intermolecular contacts are shown and side chain to side chain hydrogen bonds are shown as dashed lines.
