Fig. 4.
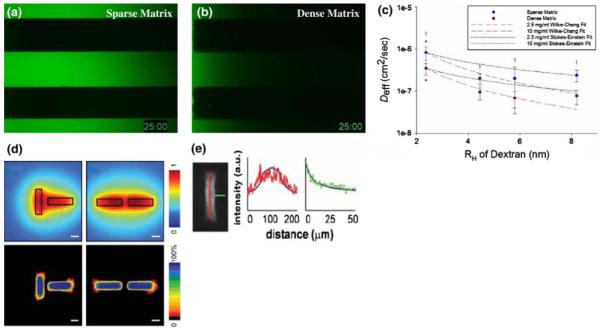
ECM composition and tissue architecture bias soluble factor gradients. To study the effects of ECM density on the passive diffusion of signaling molecules, the transport of fluorescein-tagged 10 kDa molecular weight dextran markers was monitored in microchannels containing a sparse (2.5 mg/ml fibrin) and b dense (10 mg/ml fibrin) tissues. After 25 min, it is clear that diffusion is greatly hindered in the dense matrix. c Effective diffusion coefficients (Deff) were extrapolated from these data for a range of molecular weight markers (presented as hydrodynamic radius, RH) to demonstrate the quantitative significance of this restriction († denotes P < 0.05 when comparing sparse to dense matrix conditions). These data are bounded by two well-known models of solute transport through fluid media (dotted and dashed lines). d The architecture of a tissue can also bias gradients of soluble factors. In this case, secretion of an inhibitory factor and its diffusion through a collagenous ECM was modeled computationally for mammary epithelial cell (MEC)-seeded tubules oriented perpendicular (left column) and parallel (right column) to each other. In both cases, inhibitor concentration was predicted to reach a maxim between the tubules (top row). Functionally, this predicted that MECs would branch in regions with reduced concentrations of the putative inhibitor. Heat maps (bottom row) generated from several images of tubules arranged in the described architectures illustrate that MECs do indeed branch in this fashion. Loss- and gain-of function studies demonstrated that the inhibitor in question was TGF-β1, and e fixing and staining these tissues for TGF-β1 demonstrates that the distribution of this factor matches that predicted by the computational model. Thus, knowing the distribution of an inhibitory gradient allows us to predict where branching will occur in a tissue. Developing techniques to image gradients within live tissues would allow the investigation of how the distribution of factors critical to maintaining homeostasis are disrupted by physicochemical changes within the matrix at the onset of malignancy. a–c of this figure were reproduced, with permission from The Biophysical Society, from Ghajar et al. (2008), while d and e were reproduced, with the permission from the American Association for the Advancement of Science, from Nelson et al. (2006)
