Abstract
The importance of high TCR diversity of Treg cells for self-tolerance is poorly understood. To address this issue, TCR diversity was measured for Treg cells after transfer into IL-2Rβ-/- mice, which develop lethal autoimmunity due to failed production of Treg cells. Here we show that high TCR diversity of pre-transferred Treg cells led to selection of therapeutic Treg cells with lower TCR diversity that prevented autoimmunity. Pre-transferred Treg cells with lower diversity led to selection of Treg cells through substantial peripheral reshaping with even more restricted TCR diversity that also suppressed autoimmune symptoms. Thus, in a setting of severe breakdown of immune tolerance due to failed production of Treg cells, control of autoimmunity is achieved by only a fraction of the Treg TCR repertoire, but risk for disease increased. These data support a model where high Treg TCR diversity is a mechanism to ensure establishing and maintaining self-tolerance.
Keywords: Rodent, T cells, autoimmunity, T cell receptor, repertoire development
Introduction
The factors influencing the thymic and peripheral selection of the Treg cell TCR repertoire are of much interest as they likely represent a major control point in establishing and maintaining self-tolerance. Considerable data indicate that development of Treg cells requires recognition of self-peptide/MHC at an affinity threshold that usually falls between that required for positive vs. negative selection (1-6). Most information concerning the Treg cell TCR repertoire has come from analysis of the entire pool of Treg cells in the thymus and periphery from autoimmune-free mice and humans (4, 6-11). In the mouse studies, TCRβ transgenic mice were used so that one dominant rearranged TCR β-chain was expressed by all T cells. Analysis was then focused on the diversity of endogenous TCRα, which defined the TCR specificity. These experiments indicate that TCRs of Treg cells are highly diverse with a repertoire as broad as that found on conventional T cells. The specificities of Treg and T conventional cells were clearly distinctive, but there was some obvious overlap. Thus, the TCR specificity on developing thymocytes and subsequent selection events do not solely specify the decision to be Treg vs. T conventional cells. In addition, the TCR repertoires of thymic and peripheral Treg cells more closely resembled each other than the TCR repertoire of conventional T cells. Correspondingly, most peripheral Treg cells originate from their development and commitment to the Treg lineage within the thymus rather than from conversion of conventional peripheral T cells into induced Treg cells.
The TCR repertoire expressed by thymic Treg cells is reshaped as reflected by a reduction in the frequency of dominant thymic Treg cell TCR specificities in peripheral Treg cells (4, 6, 8-10). The mechanisms responsible for flattening the peripheral Treg cell TCR repertoire are poorly understood, but may reflect homeostatic regulation and influences of self and environmental antigens on peripheral Treg cells (10, 12). Furthermore, the TCR repertoire of peripheral Treg cells varied considerably when examined based on anatomical location of draining lymph nodes (13, 14), consistent with a role for peripheral tissue-specific self-antigens in shaping the TCR repertoire,
Given the high diversity of the Treg cell TCR repertoire, an important question is whether this high TCR diversity is mandatory to suppress potential autoreactive T cells in the periphery that escape thymic negative selection. The current study was designed to address this issue for a population of polyclonal autoreactive T cells by utilizing IL-2Rβ-deficient mice as a model. Due to the failed production of an effective population of Treg cells, IL-2Rβ-/- mice develop rapid lethal systemic autoimmunity that resembles the disease associated with Foxp3-deficient mice. Cell transfer studies indicate that this autoimmune syndrome is primarily due to autoreactive CD4+ T cells (15). The adoptive transfer of either syngeneic or even fully allogeneic WT Treg cells into neonatal IL-2Rβ-/- mice fully prevents this autoimmune disease such that the recipient mice live a normal life-span (16-18). Importantly, these donor Treg cells, including the allogeneic Treg cells, stably engraft, expand, and persist life-long through extensive homeostatic proliferation to comprise essentially the entire pool of CD4+CD25+ Foxp3+ Treg cells within these autoimmune-free IL-2Rβ-/- mice. Here we evaluated TCR repertoire diversity of such donor Treg cells when they were obtained from WT mice or mice that expressed a single TCRβ chain.
Material and Methods
Mice
C57BL/6, BALB/c, C57BL6 TCRα-/-, C57L/J-Tg(Tcrb)93Vbo/J TCRβ Tg mice (designated TCRβ Tg) (19) were obtained from The Jackson Laboratory. The TCRβ Tg mice were backcrossed to C57BL/6 and sometimes to TCRα-/- mice, the latter to yield TCRα+/- mice. CD45.1-congenic C57BL/6 mice (B6.SJL-Ptprca/BoyAiTac) were obtained from Taconic and bred in our animal colony. C57BL/6 or BALB/c IL-2Rβ-/- mice (15) were bred in our colony using autoimmune-free breeding pairs as previously described (18). Treg cells were adoptively transferred by i.v. injection into the superficial facial vein of 1-2 day-old neonatal IL-2Rβ-/- mice. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami.
Cell Purification and in vitro Treg suppression assay
CD4+CD25+ T cells were isolated by magnetic beads based fractionation as previously described (18). In some experiment, the donor Treg cells were sorted from IL-2Rβ-/- recipients by FACS to >99% purity based on expression of CD4, CD25high and Vβ8.2. Both methods yielded cells that were typically >90% Foxp3+ T cells and were more than 90% of donor origin, when purified from adoptively transferred IL-2Rβ-/- recipients. The CD25- fraction of cells from the Treg cell magnetic-beads purification was either directly used for spectratyping analysis and is referred to as conventional CD4+ CD25neg T cells or was further purified by positive selection using anti-CD4 magnetic beads for the responding cells in the Treg suppression assay, which was performed as previously described (18).
Antibodies and FACS Analysis
Biotin-conjugated mAbs to Vβ8.1/8.2 (F-23-1) and CD69 (H1.2F3), Cy-Chrome-conjugated mAbs to CD4 (H129.19), and CD8α (53-6.7), phycoerythrin (PE)-conjugated anti-CD25 (PC61), anti-H2d (SF1-1.1) and FITC-anti TCRVβ subgroups were purchased from Pharmingen. FITC-anti CD4 (GK1.5), biotin-conjugated mAbs to CD45.1 (A20), and CD25 (7D4) were prepared in our laboratory. Foxp3 expression was measured by staining with PE-conjugated anti-mouse/rat Foxp3 antibody (FJK-16s; eBioscience) according to the manufacturer's protocol. FACS analysis was performed as previously described using a LSR1 (BD Biosciences) and CellQuest software (17). 40,000-50,000 events were typically collected per sample.
CDR3 size and sequence analysis
Primers for TCR Vα and Vβ (20) spectratyping and the determination of CDR3 lengths (21) have been previously described. Primers used in this study are shown in Supplemental Table 1. RNA from purified T cells was isolated using TRIzol reagent (Invitrogen) according to manufacturer's instructions. Briefly, total RNA was incubated for 5 min at 65°C with oligo-d(T)16 primer (100 pmol) and dNTPs (10 nmol each). After cooling, cDNA was synthesized with 200U Superscript III (Invitrogen) in first-strand buffer containing 5 mM DTT (Invitrogen) and 20U RNasin (Promega) at 50° C for 1 hr. PCR was performed using one-twentieth of cDNA, 250 nM sense primers specific for Vβ 4, 7, 8.2, 8.3, 9, 11, 12 and 14, and Cβ antisense primer. Instead of Cβ primer, MCB2 primer was used for amplification of Vβ8.2 in Vβ8.2 Tg mice. Following a DNA denaturation step (1 min at 95° C), PCR conditions were 40 cycles at 95°C for 1 min, 60° C 1 min, and 72° C for 1 min with a 5 min for the last extension. The sense primers for Vα (VA1-1, VA2-1, VA3-1, VA8-1 and VA14-1) and MCA2 primer were used for PCR as described above except the annealing temperature was 55°C.
For Vβ and Vα spectratyping, 0.5 μl of the PCR reaction above was subjected to a second round of PCR for 30 cycles using the amplifications conditions described above. For Vβ spectratyping, 5'-6-FAM or 5'-PET fluorescence-labeled Jβ1.1 antisense primer (20) and the Vβ sense primer described above specific for each Vβ segment were used. 5'-fluorescence-labeled MCB3 antisense primer was used for analysis of Vβ8.2 in TCRβ Tg mice. For Vβ spectratyping, 5'-fluorescence-labeled MCA3 antisense primer and the specific Vα primer described above were used. After amplification, PCR products were diluted 1:20 or 1:40 in distilled water and 0.5 μl of the diluents was loaded to ABI Prism 3730xl DNA analyzers (Applied Biosystems). Fragment analysis sample files were analyzed with Peak Scanner™ software (Applied Biosystems).
Partial or full length TCR Vα2 genes were amplified from the RT-PCR products using the VA2-1 and MCA2 primers or the Vα2-Xho primer and Vα2-Eco primer, respectively. Partial Vα2 PCR products were cloned into pCR4-TOPO vector using TOPO TA Cloning Kit for Sequencing (Invitrogen). The full length Vα2 PCR products were digested with EcoRI and XhoI and cloned into pMI retroviral vector (22) and sequenced using a pMI sequencing primer. Clones were randomly chosen for sequencing. CDR3 sequences were analyzed by BLAST and IMGT-V-QUEST.
Data analysis
To quantify skewing of the TCR repertoire, the method of Gorochov et al (23) was used and is represented as D scores. In brief, fluorescent intensity (peak height) associated with each peak in an individual spectratyping profile was automatically measured and transferred into Excel spreadsheets. The percent representation of an individual peak height was calculated in relationship to the sum of all peaks heights in that profile. First, these calculations were performed and averaged for multiple related profiles to establish a reference profile. The samples used for the reference were the CDR3 length profiles from CD4+CD25- conventional T cells from 8 individual C57BL/6 mice which exhibited a Gaussian distribution characteristic of a highly diverse TCR repertoire. For the experimental samples, the absolute difference in these percentages at each peak was determined in comparison to the reference, summed, and then divided by two. This value represents the extent that the repertoire varied from the reference for an individual Vβ or Vα profile. The D-score represents the mean of these values for each of the 8 Vβ or 4-5 Vα segments for each experimental sample.
Statistical analysis of CDR3 sequences were performed by using EstimatorS 8.0.0 software (Codwell, R.K. ) http://viceroy.eeb.uconn.edu/EstimateS). Default shared species settings were used to calculate ACE values to estimate the number of unique Vα2 sequences for each experimental group and the Morisita-Horn sample similarity index to quantify and estimate the similarity between the Vα2 sequences between any two groups. For the Morisita-Horn Index a value of 0 indicates complete dissimilarity while 1 indicates identity. A one-way ANOVA using Tukey's multiple comparison test was used to assess differences between the test groups and control (p<0.05) for D-scores and ACE values. Unpaired one-tailed Student's t-test was used to assess health scores vs. D-scores or ACE values. P values <0.05 were considered statistically significant and are designated by * in the graphs.
Results
TCR repertoire of IL-2Rβ-deficient mice
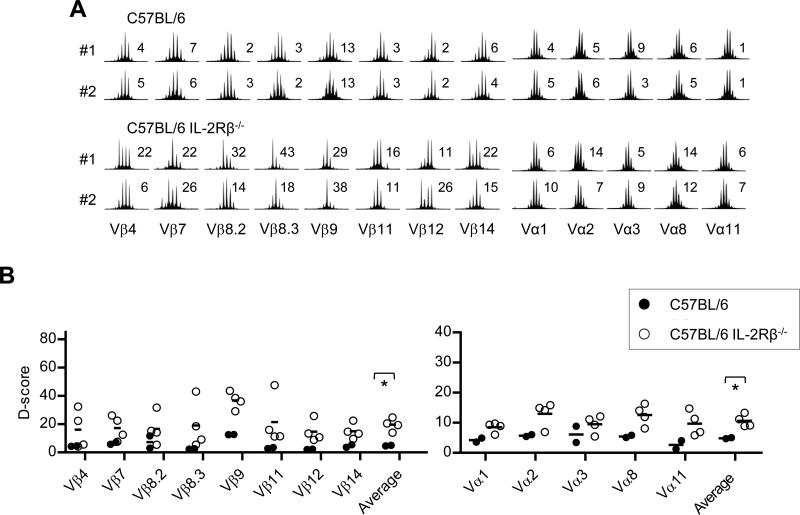
Autoimmunity and lymphoproliferative disease is evident in very young (2 wks old) IL-2Rβ-/- mice and systemic autoimmunity progresses rapidly, such that most C57BL/6 and BALB/c IL-2Rβ-deficient mice die between 8-12 and 4-6 weeks of age, respectively (18). These mice, especially early during disease, often contain lymph nodes (LN) with 10-fold increased cellularity, including autoreactive CD4+ T cells. The TCR diversity of these unregulated CD4+ T lymphocytes was broadly sampled by measuring CDR3 nucleotide lengths for several TCRβ and TCRα genes and was compared to CD4+ T cells from age-matched control WT mice (Fig. 1A). Similar to IL-2- and IL-2Rα-deficient mice (24), in most cases, a polyclonal distribution of CDR3 lengths was noted for each Vβ and Vα TCR subgroups for 3-5 week old IL-2Rβ-deficient mice (Fig. 1B), although some CDR3 length skewing was noted (e.g. Vβ8.3 for mouse #1).
Fig. 1. TCR diversity of autoreactive T cells from individual IL-2Rβ-/- mice.
CD4+ T cells were isolated from 3-5 week old WT C57BL/6 (control B6) or IL-2Rβ-/- (B6-/-) mice. (A) Representative TCR Vβ and Vα spectratype analysis for CDR3 for the indicated Vβ and Jβ1.1 gene segments or Vα subfamily and Cα gene segment. The D-score is shown to the right of each spectratype profile. (B) D-scores for Vβ and Vα spectratype distribution profiles for all mice (n=4). Data for the averaged D-scores were compared by unpaired one-tailed t-test.
To quantify these differences, diversity (D)-scores were calculated, which represent the mean of the variance of Vα or Vβ profiles from an experimental sample when compared to a highly diverse Gaussian profile from a reference sample (23), which represented the average peak height for each Vα or Vβ profile from CD4+CD25neg conventional T cells obtained from 8 adult WT C57BL/6 mice. In this report, D-scores for individual spectratyping profiles ranged from 3-5 for a naïve Gaussian fully diverse unperturbed TCR repertoire to 77-78 for a monoclonal repertoire represented by expression of a single TCRβ chain.
The D-scores for each Vα and Vβ genes were higher for IL-2Rβ-/- CD4+ T cells (Fig. 1B). When the individual Vα and Vβ D-scores were averaged, values of 10.6 and 19.6, respectively, were noted, which were significantly higher (p<0.02) than the average D-scores of 4.9 and 4.8, respectively, for WT CD4+ T cells, which closely approximated the reference samples. Very similar low D-scores were noted for conventional WT CD4+ T cells after depletion of their Treg cells (see the values for control B6 in Supplemental Fig. 3). The higher scores for IL-2Rβ-/- CD4+ T cells likely represent some clonal expansion by autoreactive T cells. Nevertheless, these data indicate that T cell lymphoproliferation that accompanies autoimmunity associated with IL-2Rβ-deficiency is not dominated by an oligoclonal CD4+ T cell response.
Health monitoring of IL-2Rβ-deficient mice
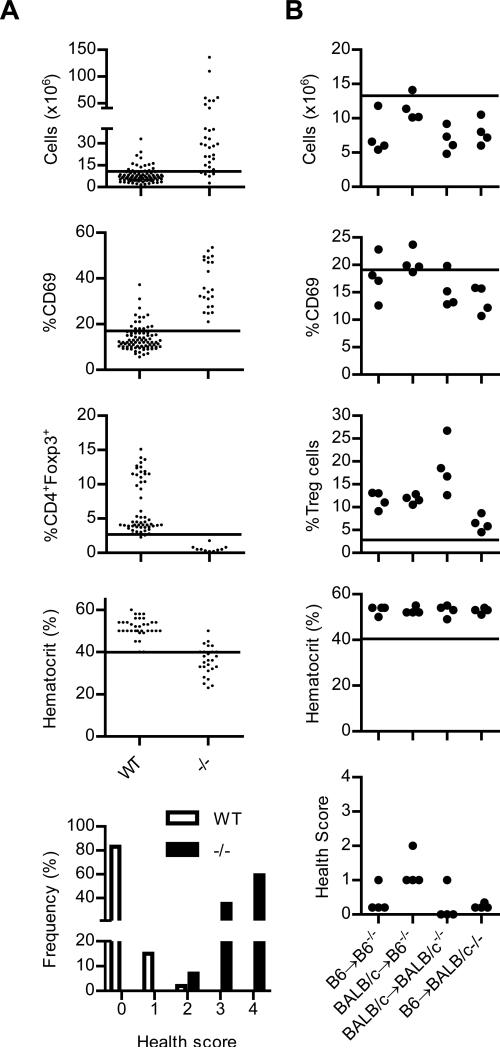
Previous data demonstrated that the adoptive transfer of 2 × 105 MHC-matched or fully mismatched donor Treg cells consistently prevented lethal autoimmune disease and the donor Treg cells persisted life-long in IL-2Rβ-/- recipient mice (16, 18). In this report our objective was to assess the TCR repertoire of such donor Treg cells in control of autoimmunity. Therefore, the autoimmune status of all IL-2Rβ-/- recipients was always assessed in parallel with TCR diversity. To establish objective criteria, we devised a health scoring system by evaluating substantial past and concurrent data from this study from normal and IL-2Rβ-/- C57BL/6 mice with regards to immunological changes that are associated with immune system dysregulation due to absent IL-2Rβ function (Fig. 2A). Early immune changes include lymphoproliferation (assessed by LN cellularity and/or histopathological evidence of lymphocytic hyperplasia) and increased activated CD4+ T cells (assessed by CD69 expression), followed by autoimmunity [assessed by low hematocrit due to hemolytic anemia (15, 18) and/or inflammatory infiltrates in non-lymphoid tissues], leading to wasting and death. IL-2Rβ-deficient mice contain only a few immature Foxp3low Treg cells in the peripheral immune compartment. For adoptively transferred mice, assessment of engraftment levels of donor Treg cells represents an additional factor related to control of autoimmunity. A cut-off for normal values was set at 1 standard deviation above or below the mean values from WT mice, as appropriate, except for the hematocrit where a more strict criteria was used for a definitive assignment of hemolytic anemia. These values are represented by the horizontal line in each graft within Fig. 2. A threshold of 1 standard deviation was chosen to establish autoimmune trends that varied from the mean rather than a more stringent threshold that would assign a high probability of an abnormal value, as these higher values generally associates with severe disease.
Fig. 2. Health status of IL-2Rβ-/- mice that were adoptively transferred with syngeneic or allogeneic Treg cells.
(A) LN cellularity, expression of CD69 by peripheral CD4+ T cells, % CD4+ Foxp3+ Treg cells in the LN, and the hematocrit were recorded from a historical data base and concurrent C57BL/6 IL-2Rβ+/+ or +/- (WT; n=99; median age, 12 wks) or IL-2Rβ -/- (-/-; n=34; median age, 4 wks) mice. 14 data points for the WT group were derived from mice 4-6 weeks of age and these also lacked an activated autoimmune phenotype. The line in each graph represents the cut-off used for normal values. The lower panel represents health scoring of these mice as described in the text of the Results. (B) 2 × 105 syngeneic or allogeneic Treg cells were adoptively transferred into 1-2 day old neonatal C57BL/6 or BALB/c IL-2Rβ-/- mice. 10-16 weeks post-transfer, the recipient mice were evaluated for the immune parameters or hematocrit, as described in (A). Based on these values, health scores were assigned for each recipient in the lower panel.
Health scores were assigned as +1 each for abnormally 1) high LN cellularity and/or histopathological evidence of lymphocytic hyperplasia in the spleen or LN, 2) increased CD69 expression by CD4+ T cells, or 3) low levels of donor Treg cells. A +4 is assigned when clear evidence of autoimmunity is observed, i.e. hematocrits below 40 and/or moderate or greater levels of lymphoplasmacytic infiltrates in non-lymphoid tissues as assigned by a veterinary pathologist. Typical targets tissues include the lung, liver, colon and salivary gland (15, 18, 25). Additional scoring include +5, weight loss of >20% and +6, death. When these criteria were applied to the groups of WT and IL-2Rβ-/- mice (Fig. 2A, bottom) used to set these thresholds, >90% of the mice scored ≤1 or ≥3, respectively. Correspondingly, a health score ≤1 is considered auto-immune-free while ≥3 is considered indicative of autoimmunity. A score of 2 indicates a trend towards autoimmunity.
These same variables were examined for C57BL/6 and BALB/c IL-2Rβ-/- mice that received syngeneic or allogeneic Treg cells (Fig. 2B) and these cells were the source of donor Treg cells for TCR repertoire analysis (see below). With the exception of one mouse with a health score of 2, all remaining recipients had health scores of ≤1 and most were 0 (Fig. 2B, bottom). The main reason for a health score of >0 was a few mice had slightly higher than normal percentage of CD4+CD69+ T cells. All these recipient mice were examined at 10-16 weeks of age, a time when most untreated IL-2Rβ-deficient mice succumb to autoimmune disease. These data, therefore, confirm the effectiveness of donor syngeneic and allogeneic Treg cell to prevent autoimmunity in IL-2Rβ-/- mice.
TCR repertoire of donor Treg cells from autoimmune-free IL-2Rβ-deficient mice
An important advantage of the IL-2Rβ-deficient model is that the donor Treg cells provide a fixed population of cells to assess their TCR repertoire as it relates to suppression of autoimmunity of polyclonal autoreactive T cells that are continually emerging from the thymus. To directly and broadly investigate TCR diversity within the donor Treg cells, the CDR3 size distribution pattern of various Vβ and Vα TCR subgroups was assessed for donor syngeneic and allogeneic Treg cells from individual recipient mice by spectratyping. Comparing the TCR repertoire of engrafted syngeneic and allogeneic donor Treg cells provided a means to assess whether clonal diversity of self-reactive Treg cells was similar or exceeded cross-reactive alloreactive Treg cells.
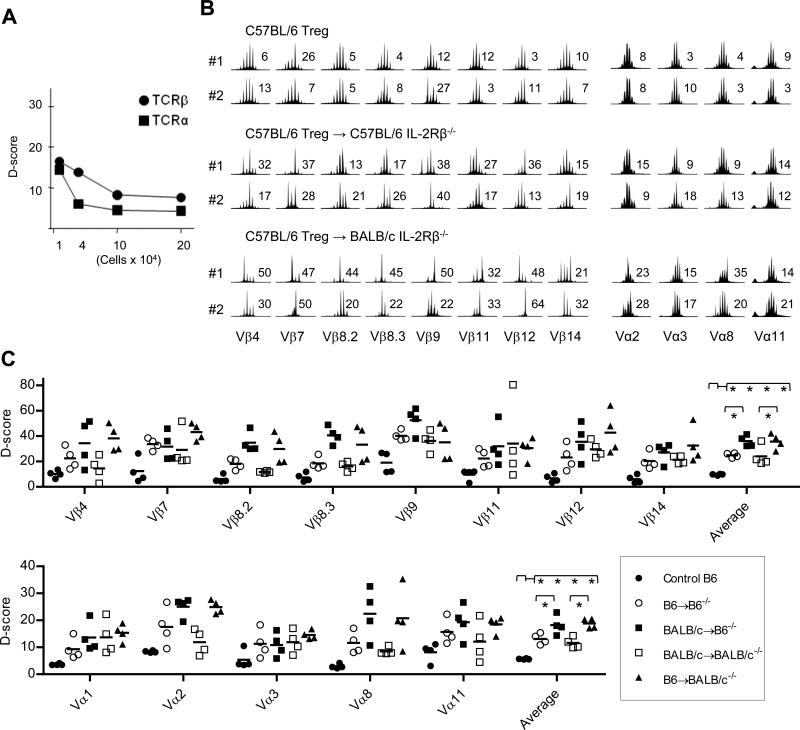
Initially, CD4+ T cells were titrated to determine the point where spectratyping profiles (Supplemental Fig. 1) began to vary from fully Gaussian. After calculating D-scores, this was 1 × 105 cells for TCRβ and 4 × 104 cells for TCRα (Fig. 3A). Spectratyping analysis of all experimental samples was performed using cell numbers that were always greater than these limits and usually >2 × 105 T cells were used as this number of donor Treg cells was typically obtained from individual recipients. Thus, a deviation from a highly diverse Gaussian spectratyping profiles cannot be attributed to insufficient sample size.
Fig. 3. TCR diversity of donor Treg cells from individual IL-2Rβ-/- mice adoptively transferred with syngeneic and allogeneic Treg cells.
(A) The sensitivity of CDR3 spectratype analysis. Vβ or Vα spectratype analysis were performed with serial diluted CD4+ T cells from a C57BL/6 mouse. Total RNA was extracted from the indicated number of cells. One-twentieth of the cDNA was used for CDR3 spectratype analysis. D-scores were calculated and plotted against cell number. Supplemental Fig. 1 shows the spectratyping profiles that were used to calculate the D-scores. (B) Spectratype analysis of CD4+CD25+ Treg cells isolated from normal C57BL/6 (B6+/+) or the adoptively transferred IL-2Rβ-/- mice analyzed in Fig. 2B. Representative Vβ and Vα spectratype analysis for CDR3 for the indicated Vβ and Jβ1.1 gene segments or Vα subfamily and Cα gene segment for Treg cells isolated from individual mice. The D-score is shown to the right of each spectratype profile. (C) D-scores for Vβ and Vα spectratype distribution profiles for all mice (n=4 mice/group). Data for the averaged D-scores were compared by 1-way ANOVA.
As expected, Treg cells directly isolated from C57BL/6 mice, which represent the input cells used for adoptive transfers, exhibited Gaussian TCRα and TCRβ CDR3 size distributions (Fig. 3B, top) and low D-scores (Fig. 3C) with an average value of 5.7 and 9.7, respectively, characteristic of a highly diverse TCR repertoire. However, TCRβ spectratyping profiles for donor C57BL/6 Treg cells isolated 10-16 weeks post-transfer from syngeneic (Fig. 3B, middle) or allogeneic autoimmune-free IL-2Rβ-/- recipients (Fig. 3B, bottom) were not Gaussian for many of the Vβ subgroups, which was more striking for donor MHC-mismatched C57BL/6 Treg cells from autoimmune-free BALB/c IL-2Rβ-deficient mice. Similar spectratype profiles were obtained for BALB/c Treg cells after adoptive transfer into syngeneic BALB/c or allogeneic C57BL/6 IL-2Rβ-/- recipients (Supplemental Fig 2). Although skewed CDR3 size distributions were noted for many Vβ subgroups, individual mice exhibited unique patterns of skewing within a particular Vβ subgroup, consistent with distinctive Treg cell TCR repertoires associated with each recipient. Such skewing of the spectratype profiles was less dramatic for TCRα.
D-score analysis of the spectratyping profiles from all 16 individual donor Treg cell samples for each Vα and Vβ subgroup revealed three obvious trends (Fig. 3C). First, D-scores from donor engrafted Treg cells were typically higher than Treg cells from normal C57BL/6 mice. Second, D-scores for donor Treg cells were generally higher for TCRβ than TCRα. Third, D-scores from donor allogeneic Treg cells were usually higher than donor syngeneic Treg cells. When considering donor C57BL/6 Treg cells from syngeneic and allogeneic IL-2Rβ-/- recipients (Fig. 3C), the averaged D-scores were 24.8 and 36.0 for TCRβ. These values were significantly higher (p<0.05) when compared to input C57BL/6 Treg cells. Averaged D-scores for TCRα were 13.1 and 18.2 for syngeneic and allogeneic donor C57BL/6 Treg cells, respectively. Nevertheless, these D-scores (p<0.05) were greater than noted for the input Treg cells, consistent with some selection of the TCRα repertoire. Very similar and statistically significant differences were noted for the average D-scores of BALB/c Treg cells from syngeneic and allogeneic IL-2Rβ-/- recipients (Fig. 3C). These results indicate that fewer alloreactive Treg cells are selected after adoptive transfer and suggest that antigen recognition plays an important role for the Treg cells that persist in the IL-2Rβ-/- recipients. Collectively, these findings indicate that a Treg cell population with measurable limitations on their TCR repertoire remains effective in preventing autoimmunity that is potentially initiated by a more diverse autoreactive TCR repertoire.
Spectratyping was also performed on recipient conventional CD4+ T cells from the autoimmune-free Treg cell “cured” IL-2Rβ-/- mice (Supplemental Fig. 3). When considering recipients T cells that received C57BL/6 Treg cells, quantitative analysis revealed averaged D-scores for TCRα and TCRβ of 8.5 and 14.7, respectively, in a syngeneic setting (C57BL/6 IL-2Rβ-/- recipients) and 7.9 and 16.2, respectively, in an allogeneic setting (BALB/c IL-2Rβ-/- recipients). These values are lower than found in untreated IL-2Rβ-/- mice, but significantly higher (p< 0.05) for TCRβ, but not TCRα, when compared to conventional CD4+ T cells from normal mice. Very similar averaged D-scores and trends were noted for recipient T cells that received BALB/c Treg cells. These results demonstrate equivalent degree of normalization of TCR repertoires in these IL-2Rβ-deficient recipients by syngeneic and allogeneic Treg cells. This finding is consistent with the ability of syngeneic and allogeneic Treg cells to readily suppress autoimmunity.
Control of autoimmunity by Treg cells with a single TCRβ
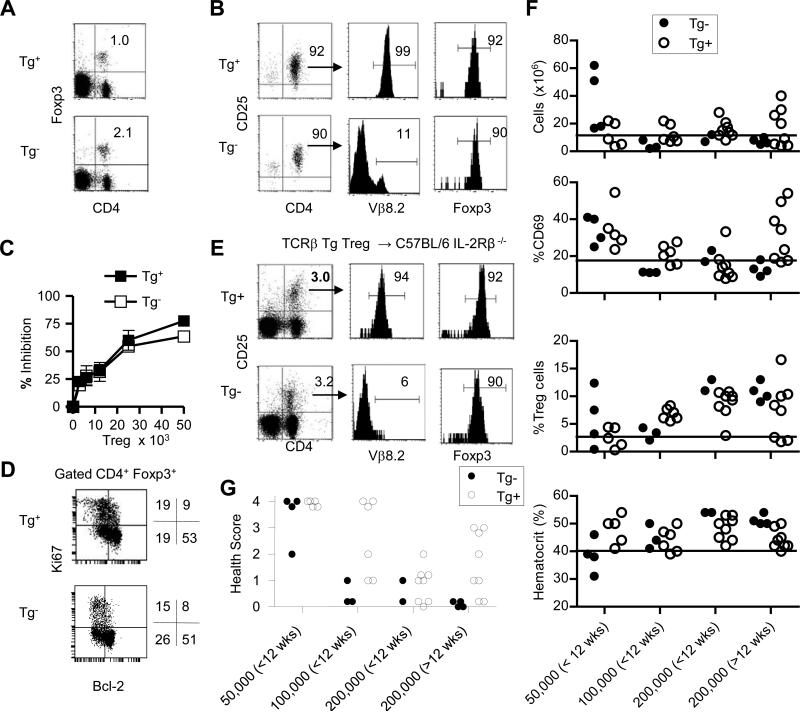
TCRβ Tg mice provide a source of Treg cells with substantial limits on their TCR diversity due to allelic exclusion at the TCRβ locus as it results in T cells that express a single TCR β-chain. Direct analysis of cells from these mice indicates that their decreased TCR diversity resulted in selection of a lower proportion of Treg cells within the peripheral immune compartment when compared to littermate control mice (Fig. 4A). FACS analysis confirmed that purified Treg cells from TCRβ Tg+ mice on the H-2b genetic background dominantly expressed a single TCRβ chain on essentially all CD4+CD25+ Foxp3+ Treg cells (Fig. 4B). When TCRβ Tg+ and Tg- littermate control Treg cells were assessed in vitro, both types of Treg cells equivalently suppressed T cell proliferation, indicating that Treg cells selected through a single TCRβ chain did not exhibit any intrinsic loss of suppressive activity (Fig. 4C). Treg cells with a single TCRβ chain are under similar homeostatic regulation in the periphery as Tg- C57BL/6 Treg cells as assessed by Ki67, a molecule expressed from mid G1 through G2M phases of the cell cycle, and Bcl-2 staining of Foxp3+ T cells (Fig. 4D).
Fig. 4. TCRβ Tg+ Treg cells as a donor cells for adoptive transfer into neonatal C57BL/6 IL-2Rβ-/- mice.
Treg cells were purified from the spleens of TCRβ Tg+ and Tg- mice and adoptively transferred into neonatal C57BL/6 IL-2Rβ-/- mice. Pre-transferred (A-D)Tg+ and Tg- Treg cells were enumerated before purification (A) and after purification (B). Expression of Vβ8.2 and Foxp3 was examined for the purified cells (B) contained within the upper right quadrant. (C) Inhibition of anti-CD3-induced proliferation of conventional CD4+ T cells by pre-transferred TCRβ Tg+ and Tg- Treg cells. (D) Expression of Ki67 and Bcl-2 by CD4+ Foxp3+ Treg cells prior to adoptive transfer. (E) Representative engraftment by donor TCR Tg+ and Tg- Treg cells 8 wks post-transfer from IL-2Rβ-/- recipients that received 2 × 105 Treg cells. (F) LN cellularity, expression of CD69 by peripheral CD4+ T cells, Treg cells in the LN, and the hematocrit were assessed for individual IL-2Rβ-/- recipients at 8-18 wks post-transfer. The x-axis represents the Treg input dose and the time post-transfer the recipients were analyzed. The line in each graph is from Fig. 2A and represents the cut off for normal values. (G) Based on the values in (F) and histopathology, health scores were assigned for each recipient. Health scoring for recipient of Tg- donor Treg cells includes relevant mice from Fig. 2 and additional recipients. The numbers within the gated regions (A,B, E) or to the right of the dot plots (D) represent the percent positive cells.
To directly explore the efficacy of Treg cells with a limited TCR repertoire to prevent autoimmunity, we assessed the ability of graded numbers of WT and TCRβ Tg+ Treg cells to engraft and control autoimmunity in IL-2Rβ-/- mice. To follow engraftment we took advantage of past work that demonstrated that essentially all Treg cells in the adoptively transferred IL-2Rβ-/- mice are of donor origin (16-18). Indeed, a representative example of such an autoimmune-free recipient shows that approximately 98% of the Foxp3+ T cells were CD45.1+ congenic-marked donor Treg cells that were all CD25high (Supplemental Fig. 4). The few host Foxp3+ T cells were immature Treg cells based on lower expression of Foxp3 and lack of CD25. These features, coupled with the near ubiquitous express of the Vβ8.2 of the TCRβ Tg+ Treg cells, allowed identification and isolation of donor TCRβ Tg+ Treg cells by selection of CD4+CD25+ T cells.
With respect to engraftment, donor MHC-matched TCRβ Tg+ Treg cells were readily detected in the periphery of C57BL/6 IL-2Rβ-/- mice that received 2 × 105 Treg cells at a level similar to found for control Tg- donor Treg cells (Fig. 4E). Furthermore, engraftment of TCRβ Tg+ Treg cells 1 week post-transfer was comparable (data not shown) to that previously found for WT Treg cells (17), indicating that restricting Treg cell TCR diversity did not affect early steps controlling engraftment and expansion of the donor Treg cells. For the recipients of TCRβ Tg+ Treg cells, >90% of the their CD4+CD25+ T cells were Vβ8.2+ and Foxp3+ whereas Vβ8.2+ TCRs were expressed on a small minority of the engrafted WT donor CD4+CD25+Foxp3+ T cells (Fig. 4E). Engraftment by TCRβ Tg+ Treg cells was similar in most IL-2Rβ-/- recipients that received 2 × 105 donor cells (Fig. 4F) and at a proportion of the CD4+ T cells typically seen when WT Treg cells are transferred. However, TCRβ Tg+ Treg cells engraftment was dose-dependent with lower proportional engraftment at lower number of input Treg cells (Fig. 4F).
With respect to health status, there was an obvious correlation between number of input donor TCRβ Tg+ Treg cells, the subsequent proportional engraftment, and abnormal measurements of individual parameters of health status (Fig. 4F). This is particularly evident when examining CD69 expression on CD4+ T cells for recipients that received 0.5-2 × 105 donor Treg cells (Fig. 4F). Of note for the 200,000 >12 wk input TCRβ Tg+ group, the 3 mice with the highest %CD69+ were the same 3 mice with the lowest %Treg cell engraftment (Fig. 4F). Although very few of these mice had abnormal readings for hemolytic anemia, extensive histopathogy was performed on most of these recipients as another measure of autoimmunity and used in health scoring.
A dose-dependent relationship was noted where at 0.5 × 105 donor input Treg cells, most recipients of WT or TCRβ Tg+ Treg cells exhibited autoimmunity, while both types of Treg cells controlled autoimmunity at 1-2 × 105 donor input cells (Fig. 4G). The parallel loss of suppression of autoimmunity by 0.5 × 105 WT or TCRβ Tg+ input Treg cells indicates that factors other than TCR diversity contribute to prevent autoimmunity and this result may be due to an initial lower ratio of Treg cells to autoreactive T cells, such that the autoreactive T cells prevailed. This conclusion is supported by other work that showed multi-organ inflammatory disease occurred in settings where lymphopenic mice are reconstituted with T cells populations containing limiting number of Treg cells (26). For IL-2Rβ-/- recipients of 2 × 105 Treg cells, 18/18 and 11/16 that received WT (Figs. 2B and 4G) and TCRβ Tg+ (Fig. 4G) Treg cells, respectively, were autoimmune-free (health score ≤1). Thus, in a model where autoimmune disease penetrance is 100% for untreated mice, a typical curative number of donor Treg cells with a single TCRβ chain, which inherently constrains the TCR repertoire, often prevents autoimmunity. Nevertheless, a tendency of higher health scores was assigned to recipients that received 1-2 × 105 TCRβ Tg+ Treg cells. This finding suggests that the constraints of a single TCRβ chain may lead to “holes” in the Treg cell TCR repertoire that act as an initiating factor for autoimmunity. The strong association of MHC or HLA polymorphisms and autoimmune diseases may in part reflect such a contraction of the Treg cell TCR repertoire.
The TCRα repertoire of donor Treg cells expressing a single TCRβ chain
The donor TCRβ Treg cells were isolated from most of the recipient IL-2Rβ-/- mice to evaluate the diversity of their TCR α-chains. Spectratyping was performed for Treg cells from recipients that received 50,000 and 200,000 Treg cells to broadly assess the extent TCR diversity was narrowed under conditions of suboptimal vs. optimal number of transferred cells and as a function of disease status. Representative spectratype profiles are shown for the input TCRβ Tg+ Treg cells (Fig. 5A, top), selected mice that were autoimmune-free (Fig. 5A, middle), or exhibited autoimmunity (Fig. 5A, bottom). The input T cells expressed a generally polyclonal endogenous TCRα repertoire [(19) and Fig. 5A, top], with an averaged D-score of 18.4 (Fig. 5B). Furthermore, direct analysis of conventional CD4+ T cells from these TCRβ transgenic mice revealed an averaged D-score of 22.8 (Supplemental Fig. 5). Both these values were higher than found for WT C57BL/6 Treg or T conventional cells (D-scores typically 5-6) and likely reflect some limitation for TCRα pairing with a single TCRβ chain.
Fig. 5. Spectratype analysis for TCRα diversity of input and donor TCRβ Tg+ Treg cells from individual IL-2Rβ-/- recipient mice.
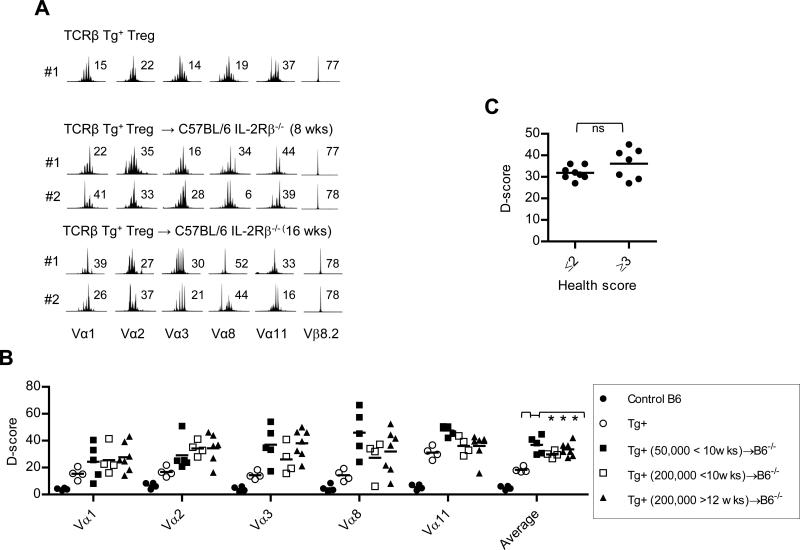
At the indicated time post-transfer, TCRβ Tg+ Treg cells were purified from individual C57BL/6 IL-2Rβ-/- recipients. (A) Representative Vα spectratype analysis for CDR3 was performed for the indicated Vα subfamily and Cα gene segment for input and donor-derived Treg cells. Spectratype analysis for CDR3 size distribution of Vβ8.2 is shown to illustrate the expression of the TCRβ Tg by these populations of purified Treg cells. The D-score is shown to the right of each spectratype profile. (B) D-scores for Vα spectratype distribution profiles for all mice (n=4-6 mice/group). Data for the averaged D-scores were compared by 1-way ANOVA. (C) The averaged D-score for the recipients that received TCRβ Tg+ Treg cells were plotted against the health scores as assigned in Fig. 4F. Data were compared by unpaired one-tailed t-test.
Both the input and engrafted Treg cells showed a single monoclonal peak and a D-score of 77-78 for Vβ8.2, further confirming their high purity and donor origin (Fig. 5A). The engrafted Treg cells 8-16 wks post transfer showed more obviously skewed Vα spectratyping profiles (Fig. 5A, middle & bottom) and this skewing varied for Treg cells from individual recipients, e.g. the profiles for Vα1 and Vα8. Importantly, quantitative analysis of these profiles from all individual mice revealed a consistent trend of higher D-scores for each of 5 Vα subgroups that in aggregate assessed approximately 30% of the Vα genes (Fig. 5B). The averaged D-scores for the engrafted Treg cells (29.8-36.6) were higher and significantly different (p <0.05) from the input Treg cells but not from each other. This finding suggests that the Treg TCR diversity is independent of the number of transferred Treg cells and health status of the recipient. Indeed, there was no significant difference in the mean averaged D-scores when they were plotted as a function of health scores (Fig. 5C).
D-score analysis for the same Vα subregions was also performed for conventional CD4+ T cells from several IL-2Rβ-deficient recipients at <10 and >12 weeks post-transfer that were found to be autoimmune-free or diseased, respectively (Supplemental Fig. 5). Averaged D-scores lower than found for untreated IL-2Rβ-/- mice were only seen for the auto-immune-free IL-2Rβ-/- recipients. This result provides additional support for control of autoimmunity by TCRβ Tg+ Treg cells. Collectively, these experiments illustrate that Treg cells with the constraints of a single TCR β-chain and restricted TCRα diversity suppress polyclonal autoimmunity associated with IL-2Rβ-deficient mice, but control of this autoimmunity is not solely related to the diversity of these donor Treg cells.
CDR3 sequences of TCRα of donor Treg cells that express a single TCRβ chain
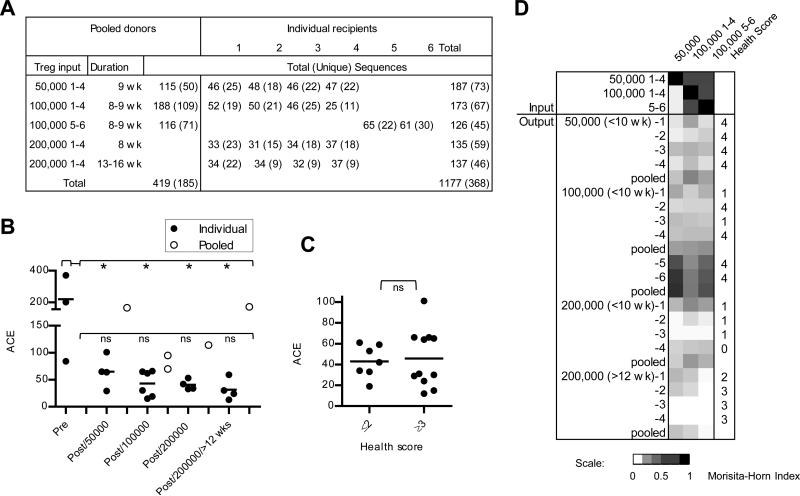
To further evaluate the restriction of the TCRα repertoire and specificity, CDR3s from Vα2 TCRs were sequenced from 3 pools, each derived from 5-6 mice, of pre-transferred input donor Treg cells and 18 individual recipients at 8-16 weeks post-transfer (Fig. 6A). For each experiment according to the shown donor/recipient relationship, 100-200 sequences were obtained for each pool of input Treg cells while usually 30-50 sequences were obtained from recipient-derived Treg cells such that when pooled each group also consisted from 100-200 sequences. The numbers of TCRs in each individual sample or after pooling the sequences within a group were calculated using the abundance-based coverage estimator (ACE) of species richness (Fig. 6B). This analysis revealed statistically significant (p<0.05) higher ACE values, indicative of greater diversity, for the Vα2 TCRs from the pre-transferred donor Treg cells when compared to those for the post-transferred cells from individual recipients. These latter values were not significantly difference from each other. A plot of ACE values and health scores of the corresponding individual recipient mice revealed a trend toward lower ACE values and higher health scores, but this was not statistically significant. (Fig. 6C), which agrees with the spectratyping results. Collectively, these data indicate that each recipient contains TCRs with more limited diversity than the input donor cells and that factors other than TCR diversity also importantly determine whether autoimmunity occurs. One such factor may be the specificities of the Treg TCRs present in each recipient.
Fig. 6. Vα2 CDR3 sequence diversity and similarity from donor TCRβ Tg+ Treg cells pre- and post-transfer into IL-2Rβ-/- recipient mice.
CDR3 sequences for Vα2 TCR subfamilies were determined for Treg cells for recipients analyzed in Fig. 4 and 5. The input pre-transferred Treg cells were typically pooled from 4-6 mice to acquire sufficient cells to inject all neonatal IL-2Rβ-/- mice from a litter. The indicated pre- and hence post-transferred TCRβ Tg+ Treg cells are on the TCRα+/- genetic background to minimize sequences from Treg cell that expressed two TCR α-chains. Treg cells from the other recipients are on the TCRα+/+ genetic background. (A) Sequence accumulation of pre- and post-transferred Treg cells where recipients from an individual litter are grouped. (B) The number of sequences within individual samples of pre-transferred Treg cells or isolated from individual recipients was calculated by ACE and compared by 1-way ANOVA. A similar ACE calculation was made after grouping together (pooled) the entire set of sequences from the same litter of recipients as these mice received the same pre-transferred Treg cell inoculum. The two pooled samples at post/100,000 represent individuals 1-4 and 5-6. (C) ACE values for the Treg cells from individual recipients were plotted against the health scores as assigned in Fig. 4F. Data for the ACE values were compared by unpaired one-tailed t-test. (D) The similarity of CDR3 sequences of the pre-transferred Treg cells was compared to all post-transferred cells by calculation of Morisita-Horn similarity values and represented by a heat map. Input sequences were compared to sequences from individual recipients or as pooled groups based on receiving the same donor inoculums.
The lower ACE values for TCR diversity of Treg cells that engrafted and persisted within each recipient was independent of input cell number for the range tested. However, after the Treg cell TCR sequences from each individual recipient within a group were pooled, the ACE values consistently increased (Fig. 6B). This finding suggests that overall fraction of Treg cell specificities that contribute to the control of autoimmunity is greater than operative within a single IL-2Rβ-deficient recipient.
Morisita-Horn similarity values were also compared for these groups of TCR data sets pre- and-post transfer (Fig. 6D). This analysis revealed that TCR sequences for the input pre-transferred Treg cells were more similar to each other than to 16 of 18 post-tranferred Treg TCR sequences, either when analyzed individually or pooled. Two exceptions were recipients 5 and 6, which received the same distinct group of donor Treg cells. Higher similarity to the input Treg cells was noted, but both recipients exhibited a high health score of 4, indicative of autoimmunity. The lower overall similarity for recipients that received 200,000 donor Treg cells may reflect in part that TCR sequences were not obtained from the pre-transferred cells for these two groups. Furthermore, low Morisita-Horn similarity values were noted for the individual Treg cell TCRs post-transfer when compared to each other, even between individuals that received the same inoculums of donor Treg cells (Supplemental Fig. 6).
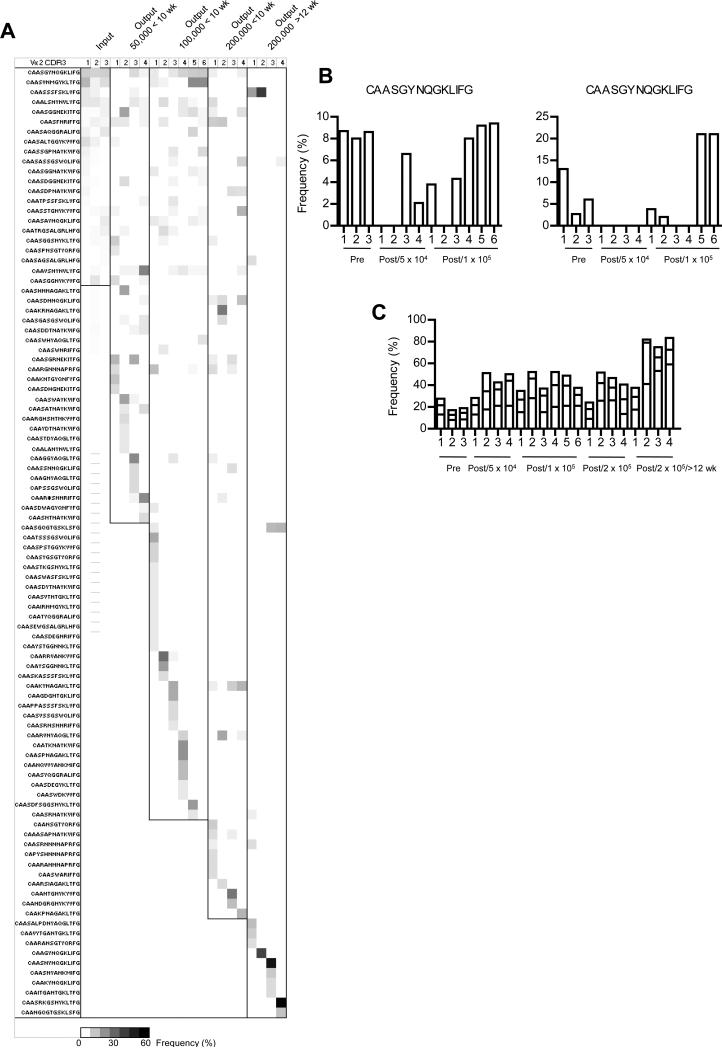
The relationship between the Treg TCR specificities were compared for any CDR3 for an individual pre-and post-transfer sample that was detected at a frequency of ≥4%. An overlap was found in sequences for the pre- and post-transferred donor Treg cells (Fig.7A) and sometimes the most highly frequent sequence was associated with the post-transferred Treg cells. The two most prevalent sequences in the input pre-transferred Treg cells were not always found in the post-transferred cells (Fig. 7B). Moreover, a large majority of these prevalent sequences (74 of 96) were detected only from the post-transferred donor Treg cells. Some of these sequences were shared between donor Treg cells from distinct recipients, but most were not. Many of the most frequent post-transferred donor Treg TCR CDR3s were unique to particular recipients. For a sequence at a frequency of 5% in the post-transferred Treg cells, binomial probability calculations indicate that there is >99% confidence level that this sequence would be found in the pre-transferred cells when accumulating 115-188 CDR3s. Considering this calculation and the most prevalent sequence associated with post-transferred Treg cells from individual recipients was found at frequency usually ≥15% (Fig. 7C), many of these dominant specificities must have been derived from a minor constituent of the pre-transferred Treg cells. In comparison to the input Treg cells, more highly frequent Vα2 sequences were associated with most of the post-transferred Treg cells that was particularly striking in the 3 of the 4 mice each with health scores of 3 that received 200,000 Treg cells and evaluated 13-16 weeks post-transfer (Fig. 7C). Collectively, these results indicate that there is substantial TCR repertoire reshaping after adoptive transfer of Treg cells into IL-2Rβ-deficient mice and at sufficient input cell number (>100,000 Treg cells) control of autoimmunity is often still achieved.
Fig. 7. Frequency of Vα2 CDR3 sequences associated with pre- and post-transferred TCRβ Tg+ Treg cells.
(A) All unique sequences in the Vα2 data set expressed at a frequency ≥4% in any pre-transferred sample (input) or any individual recipient post-transferred (output) were identified. The prevalence of these sequences were compared to each other and represented as a heat map. (B) The frequency distribution of the two most prevalent sequences in the pre-transferred Treg cells in relationship to matched output post-transferred Treg cells. (C) Frequency of the 3 most prevalent sequences within individual samples of pre- and post-transferred Treg cells.
Discussion
Although the Treg cell repertoire is highly diverse (7-11), the relevance of this high TCR diversity in suppressing potentially autoreactive T cells that escape thymic negative selection is poorly understood. One major finding in this report is that control of autoreactive T cells is readily achieved by only a fraction of the total TCR diversity expressed by Treg cells. We show that a fixed population of input donor Treg cells is selected upon adoptive transfer into IL-2Rβ-/- mice to express a restricted portion of the total Treg cell TCR repertoire and such Treg cells effectively suppress a continual source of autoreactive polyclonal T cells that escape thymic negative selection. This was strikingly shown when WT Treg cells were adoptively transferred into IL-2Rβ-deficient mice. All such recipients were judged to be autoimmune-free, but the donor Treg cells exhibited readily measurable limitations on their TCR diversity when compared to the input donor pre-transferred Treg cells. These limitations were broadly seen over multiple Vα and Vβ CDR3s and was particularly striking for allogeneic donor Treg cells. Moreover, control of autoimmunity also often occurred after transfer of an optimal number of 2 × 105 Treg cells with a defined limitation on their TCR diversity through expression of a single TCR β-chain, although the durability of suppression appears lower than WT Treg cells.
Post-transferred WT Treg cells showed limitations of their TCR diversity that was more striking when compared to CD4+ T cells from autoimmune untreated IL-2Rβ-/- mice, suggesting that Treg cells may require less TCR diversity than the target autoreactive cells that they suppress. In support of this idea, donor Treg cells with a single TCRβ also readily suppressed autoimmunity in IL-2Rβ recipients. We assume that the large majority of the Treg cells that persist in these recipients are dedicated to suppress autoreactive T cells. However, it is possible that the therapeutic Treg cells are a subset of the persistent engrafting cells. If this proves to be true, even more limited TCR diversity than we have measured here is sufficient for suppression of peripheral polyclonal autoreactive T cells. Of note, the calculation for diversity for all Vα2 CDR3 sequences from post-transferred TCRβ Tg+ Treg cells derived from the entire group of recipients that received a common pool of Treg cells was greater than measured for Treg cells from a single recipient. Thus, the sum of Treg cell specificities in the donor population used to suppress autoreactive T cells is greater then found in an individual recipient.
Why is the Treg cell repertoire highly diverse when only a fraction of this diversity is required to effectively suppress self-reactive T cells? One obvious answer to this question and supported by our results is that high Treg cell TCR diversity guarantees that self-tolerance is readily established toward a random conventional TCR repertoire with unpredictable and distinctive self-specificities that are not deleted as a course of central tolerance. Such a mechanism likely reflects an essential step during the evolution of the adaptive immune system to ensure self-tolerance at a very high frequency for individuals of a species. High TCR diversity by Treg cells also likely ensures their participation in regulating immune responses to various non-self antigens. Indeed, there is considerable data that Treg cell suppressive activity is not limited to auto-aggressive T cells but also down-regulates many conventional immune responses to foreign and tumor antigens (27-29).
A second major finding is that there is substantial peripheral reshaping of the Treg TCR repertoire in IL-2Rβ-deficient recipients and such reshaping varies for each recipient. This notion was suggested by spectratying of post-transferred WT Treg cells as the resulting profiles varied for TCR Vβ subgroups when isolated from distinct recipients. Vα2 sequences of pre- and post-transferred TCRβ Tg+ Treg cells indicate that this reshaping often involves selection of rare and distinct specificities within the input Treg population by each recipient, often at the expense of the dominant specificities found on the pre-transferred Treg cells. Thus, distinct pools of input Treg cells showed greater similarity to each other than when compared to virtually all post-transferred donor cells from individual recipients.
Peripheral reshaping of Treg TCR repertoire has been previously noted (8-10) and represented by a lowering in the periphery of those specificities that were dominant within the thymus. Although we often noted a decrease in the dominant specificity of the pre-transferred Treg cells, the peripheral reshaping within IL-2Rβ recipients was much more extensive and characterized by selection and expansion of minor specificities. It is intriguing to speculate that this latter process may exaggerate peripheral reshaping that normally occurs where flattening of the dominant Treg TCR specificity must lead to some favoring of other less represented specificities. An important distinction of IL-2Rβ-deficient mice is that they lack mature Treg cells (30). Upon transfer of CFSE-labeled Treg cells into IL-2Rβ-/- mice, a minor fraction of the initial donor inoculum of 2 × 105 Treg cells successfully engraft, rapidly expand to normal levels, where the CFSE-label was nearly fully diluted 7 days post-transfer (17). These donor cells provide a life-long pool of Treg cells through extensive homeostatic proliferation that function to prevent autoimmunity (17, 18, 30). Therefore, this intense homeostatic pressure likely influences the selection of a persistent pool of Treg cells. Our data rule out that the selection and reshaping of the Treg TCR repertoire is strictly a random process of homeostatic proliferation by the donor Treg cells. In this case, we expected that the TCR repertoire of post-transferred Treg cells to closely mirror the input cells. Furthermore, simply the presence of a normal proportion of Treg cells within IL-2Rβ recipients that received TCRβ Tg+ Treg cells did not predict control of autoimmunity. Thus, specificities associated with the Treg cells clearly matter to prevent autoimmunity. Consistent with this notion, the more limited TCR diversity of engrafted allogeneic Treg cells when compared to syngeneic Treg cells suggests that the former cells were selected on alloantigen. Considering these points, repertoire reshaping, although influenced by homeostasis, likely also includes indexing in some manner to self-antigen(s) and/or the autoreactive T cells, whose specificities appear to vary between individual IL-2, IL-2Rα (24), and IL-2Rβ-deficient mice (Fig. 1).
In conclusion, our data favor a model where Treg TCR specificities are actively selected and reshaped in the periphery to favor specificities to optimally suppress autoreactive T cells. This mechanism does not normally represent a risk, but rather a benefit to maintain self-tolerance, due to the high diversity of the Treg cell TCR repertoire. Thus, peripheral Treg cell TCR repertoire reshaping represents a feature of adaptive immunity to maintain tolerance as thymic output wanes or during insults to the immune system. In the latter situations, the immune system must be continually rebalanced after infections, bone marrow transplantation, or the use of drugs for immunosuppression and tumor chemotherapy. In settings where the diversity of Treg TCRs is limited, such as Treg cell transfers into IL-2Rβ-/- mice, these selective pressures may result in a key specificity to become underrepresented or absent, leading to autoimmune attack. In an analogous fashion, a similar risk for autoimmune disease may emerge in pathological and therapeutic conditions that cause T cell lymphopenia or in later life where thymic output is minimal resulting in a high reliance on Treg cell specificities of the existing pool of peripheral Treg cells.
Supplementary Material
Acknowledgements
We thank Aixin Yu and LinJian Zhu for technical assistance and Guoyan Chen for help with Ki67 analysis of Treg cells.
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in the Journal of Immunology (The JI). The American Association of Immunologists, Inc (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not been copyedited or subjected to editorial proofreading by the JI; hence it may differ from the final version published in the JI (online and in print). AAI (the JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institute of Health or any other third party. The final, citable version of record can be found at www.jiimmunol.org
This work was supported by the National Institutes of Health grant R01 AI055815.
Disclosures
The authors have no competing financial interests.
References
- 1.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 3.Picca CC, Larkin J, 3rd, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunol. Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 6.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasow KA, Chen X, Knowles J, Wichlan D, Handgretinger R, Riberdy JM. Human CD4+CD25+ regulatory T cells share equally complex and comparable repertoires with CD4+CD25- counterparts. J. Immunol. 2004;172:6123–6128. doi: 10.4049/jimmunol.172.10.6123. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 9.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J. Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 11.Fazilleau N, Bachelez H, Gougeon ML, Viguier M. Size and diversity of CD4+CD25high Foxp3+ regulatory T cell repertoire in humans: evidence for similarities and partial overlapping with CD4+CD25- T cells. J. Immunol. 2007;179:3412–3416. doi: 10.4049/jimmunol.179.6.3412. [DOI] [PubMed] [Google Scholar]
- 12.Correia-Neves M, Waltzinger C, Mathis D, Benoist C. The shaping of the T cell repertoire. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 13.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J. Exp. Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J. Exp. Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 16.Adeegbe D, Bayer AL, Levy RB, Malek TR. Allogeneic CD4+CD25+Foxp3+ T regulatory cells suppress autoimmunity while establishing transplantation tolerance. J. Immunol. 2006;176:7149–7153. doi: 10.4049/jimmunol.176.12.7149. [DOI] [PubMed] [Google Scholar]
- 17.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J. Exp. Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 19.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor β gene prevents expression of endogenous β genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 20.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor β chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. U S A. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsutani T, Ohmori T, Ogata M, Soga H, Kasahara S, Yoshioka T, Suzuki R, Itoh T. Comparison of CDR3 length among thymocyte subpopulations: impacts of MHC and BV segment on the CDR3 shortening. Mol. Immunol. 2007;44:2378–2387. doi: 10.1016/j.molimm.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Deftos ML, He YW, Ojala EW, Bevan MJ. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debre P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, Sharma R, Kung JT, Deshmukh US, Jarjour WN, Fu SM, Ju ST. Pervasive and stochastic changes in the TCR repertoire of regulatory T-cell-deficient mice. Int. Immunol. 2008;20:517–523. doi: 10.1093/intimm/dxn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc. Natl. Acad. Sci. U S A. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 28.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Re.v Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 29.Wang HY, Wang RF. Regulatory T cells and cancer. Curr. Opin. Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.