Abstract
Immunoregulatory effects of placental extract and placenta-derived factors have been demonstrated in various conditions. Accordingly, placental extract has been used as certain types of medical intervention in Asian countries, whereas experimental evidence supporting its therapeutic effects and mechanisms has yet to be fully demonstrated. In this study, we investigate preventive and therapeutic effects of placental extract in contact hypersensitivity (CHS), a mouse model of allergic contact dermatitis. Administration of placental extract prior to the sensitization of allergic antigen (Ag) significantly inhibited the severity of CHS induced by Ag challenge. This effect was associated with reduced numbers of CD4+ T cells in peripheral blood, decrease of tissue-infiltrating lymphocytes, and preferential production of Th2-type cytokines in Ag-challenged sites. In addition, CHS caused by repetitive challenges of allergic Ag was also prevented and treated by administration of placental extract. Finally, administration of cyclo-trans-4-Lhydroxyprolyl-L-serine, a dipeptide derived from placental extract, also alleviated CHS, suggesting its potential role in the effects of placental extract in CHS. Taken together, our findings demonstrated experimental evidence supporting immunoregulatory effects of placental extract in allergic skin diseases and elucidated its potential mechanisms.
Keywords: Placental extract, Contact hypersensitivity, Inflammatory skin disease, Cytokines
Introduction
There are accumulating studies demonstrating that human placenta has multiple functions as a source as well as a target of numerous biologically active molecules [1-3]. Known biological effects of human placenta and its extract include, but are not limited to, modulation of immune responses, protection and regeneration of hepatocytes, regulation of hormonal balance in women, neurological effects on brain monoamine oxidase activity, anti-coagulation, and facilitation of wound healing and pigmentation [4-9]. Consistently, a large number of growth factors, their receptors, and other biological regulators have been identified in human placenta [1, 10-14]. In clinical settings, human placental extracts have been approved and used as therapeutic drugs to improve chronic liver dysfunction and menopausal symptoms in several Asian countries [6, 15].
Immunomodulatory effects of human placental extract have been demonstrated in multiple studies. Consistent with a crucial role of placenta in generation and maintenance of fetal-maternal tolerance, prevailing data indicate that placental extract has immune-inhibitory effects through various mechanisms. Lymphocyte responses to mitogenic stimuli are inhibited by the addition of placental extract in a dose-dependent manner, mainly due to a cytostatic effect [16]. Allogeneic mixed lymphocyte reaction and graft-versus-host disease is also inhibited by placental extract [17, 18], suggesting that T lymphocyte is a potential target of the inhibitory effects. In addition, B cell functions could be also affected, as antibody (Ab) responses to the immunized antigen (Ag) are suppressed by placental extract [19]. Inflammation caused by carrageenan or other pro-inflammatory reagents was also alleviated by placental extract injections [4], further indicating its effects to modulate innate immune functions. In a therapeutic perspective, efficacy of placental extract to treat pelvic inflammatory disease has been demonstrated [20]. On the other hand, several studies have suggested immune-stimulatory, rather than immune-inhibitory, effects of placental extract, e.g. stimulation of IL-8 production from monocytic cell line via activation of JNK/SAPK pathway, enhanced induction of superoxide radicals from neutrophils, and promotion of protective immunity against visceral leishmaniasis [21-23]. These seemingly controversial observations are probably due to differences in experimental or clinical conditions. Thus, it is essential to elucidate immunomodulatory effects of placental extract on the basis of immunological conditions to be treated.
Allergic contact dermatitis, one of the most common reasons for outpatient visits in dermatology, gives significant influences on a patient's quality of life [24]. It is primarily caused by a delayed-type skin hypersensitivity, in which sensitization and subsequent re-exposures to allergen elicit localized inflammatory reactions [25]. Although various medical interventions including avoidance of Ag exposure, topical and/or systemic administration of glucocorticoids are effective treatment for contact dermatitis, novel therapeutic approaches are still in great demand in certain cases in which allergens are not identified and drug tachyphylaxis is induced. Contact hypersensitivity (CHS) is a well-established experimental model to study molecular and cellular mechanisms of allergic contact dermatitis and to explore novel therapeutic approaches [26-28]. In the sensitization phase of CHS, epidermal Langerhans cells or dermal dendritic cells uptake Ag, migrate to draining lymph nodes (LN), and present Ag to T cells so as to prime them. In the elicitation phase, sensitized T cells migrate to the skin and produce inflammatory mediators in response to Ag challenge. Compelling evidence indicate that these responses are regulated by various immune regulators, including co-stimulatory signals, adhesion molecules, Th1 or Th2-type cytokines, and chemo-attractive mediators [28].
It has been known that placental extract shows therapeutic potential in dermatological diseases. Administration of placental extract treats vitiligo and promotes wound healing probably due to proliferative stimulation of melanocytes, keratinocytes, and fibroblasts [8, 9, 29, 30]. On the other hand, effects of placental extract in inflammatory and allergic skin diseases are largely unknown. In this study, we addressed immunotherapeutic potential of placental extract in CHS and explored its immunological mechanisms.
Materials and Methods
Mice and reagents
BALB/c mice were purchased from the National Cancer Institute. In all experiments, age and gender-matched 6-10 weeks-old mice were used. The mice were maintained in the animal facility under protocols approved by the Institutional Animal Care and Use Committee. Placental extract was prepared by skimming normal human placentas in acetone after removal of blood by washing with PBS (provided by Japan Bio Products Co., Ltd., Tokyo, Japan). Placentas used for extract preparation were obtained from donors with normal full-term delivery. Informed consent for providing placenta was acquired from the all donors. Donors and provided placentas were screened for HBV, HCV, HIV and Treponema pallidum, and confirmed negative in these tests. No medical history of blood transfusion and organ transplantation was confirmed in all donors. The process of placenta acquisition and extract preparation was concordant with those for Laennec, a drug of placental extracts for liver dysfunction that has been approved by Ministry of Health, Labour and Welfare in Japan. Cyclo-trans-4-L-hydroxyprolyl-L-serine (also called as JBP485), which is isolated from hydrolysates of placental extract, was prepared as previously reported [31-33].
Placental extract implantation
Mice were anesthetized by an injection of tribromoethanol (Sigma-Aldrich, St. Louis, MO). A 5 mm-wide transverse incision was made on the right lower back and the subcutaneous space was enlarged, where 3 mg of placental extract was implanted. The skin incision was closed with sutures. The mice with sham operation underwent similar incision and enlargement of subcutaneous space, followed by closing of the incision without implantation. After operation, mice were transferred to clean cages and placed under a heating lamp and monitored for recovery from anesthesia.
Administration of cyclo-trans-4-L-hydroxyprolyl-L-serine
Cyclo-trans-4-L-hydroxyprolyl-L-serine was suspended in saline at the concentration of 24 mg/ml and injected intraperitoneally at 0.5 ml per mouse. Administration was started 3 days prior to sensitization of CHS and repeated on the day of sensitization and 3 days later. As control, a group of mice was injected with saline alone in the same schedule.
Assay for CHS
CHS caused by dinitrofluorobenzene (DNFB; Sigma-Aldrich) was conducted as previously reported [34]. Briefly, the mice were sensitized by painting 20 μl of 0.5% DNFB dissolved in 4:1 (v/v) acetone-olive oil mixture on shaved abdomen. After 24 hrs, the same sensitization procedure was repeated. Five days after the first sensitization, the mice were challenged with 10 μl of 0.2% DNFB on each side of earlobes. In some experiments, the mice were exposed to the second challenge of 0.2% DNFB 3 weeks after the first challenge. After DNFB challenge, ear swelling was measured by a thickness gauge (Kafer model 21-790-1; Villingen-Schwenningen, Germany) at the level of 0.01 mm resolution. The net increase was calculated by subtracting the thickness before challenge from that after challenge in individual earlobes. Ear tissues were harvested 2 days after DNFB challenge, fixed in formalin, and embedded with paraffin. The sections were stained with hematoxylin and eosin for pathological analysis.
Flow cytometry
Spleen and peripheral blood mononuclear cells (PBMC) were harvested from mice by a standard method [35]. Single cell suspension (1 × 106 cells/sample) was first incubated with 1 μg anti-mouse FcR mAb (clone 2.4G2) in order to block non-specific FcR staining. Cells were then stained with FITC-conjugated anti-mouse CD4 mAb and PE-conjugated anti-mouse CD62L mAb (eBioscience, San Diego, CA). Data was acquired by LSR II flow cytometer (BD Biosciences, San Jose, CA) and analyzed by FlowJo software (Tree Star Inc., Ashland, OR).
Cytokine ELISA of ear tissues
After DNFB challenge, approximately half size of earlobe was harvested and weighed. The tissue was cut into small pieces and ground with pellet pestle in 0.1% Tween-20 in PBS, followed by a freeze-thaw cycle and sonication. Samples were centrifuged and the supernatants were collected for measurement of cytokines by ELISA kits specific to IFN-γ, IL-4, or IL-10 (eBiosciences). Cytokine concentrations were compensated by original weights of harvested tissues, and expressed as ng or pg of cytokine per 1 gram of tissue [36].
Statistical analysis
Statistical significance, as measured by a two-sided paired Student's t-test, was calculated using Excel v2003 based on the number of experiments indicated in the figure legends. Differences were considered to be significant at P < 0.05.
Results
Inhibition of CHS by placental extract administration
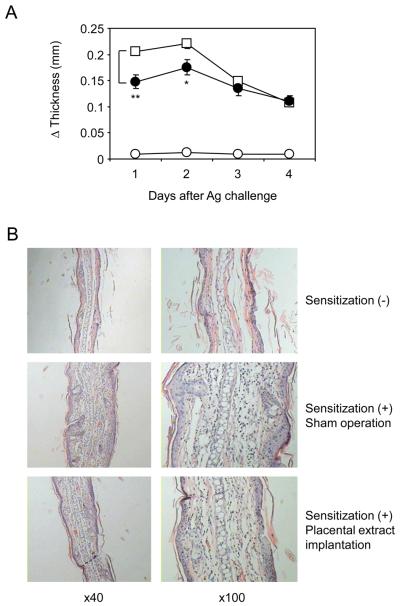
Accumulating studies have indicated potent and pleiotropic effects of placental extract in various immunological conditions [16-20]. In order to explore a therapeutic role of placental extract in skin inflammatory diseases in vivo, we employed CHS, a mouse model of allergic contact dermatitis [26-28]. Sensitization and subsequent elicitation with DNFB induced earlobe swelling, which was detectable as early as 24 hrs after elicitation and lasted for at least 4 days (Fig. 1A). This swelling was associated with immune responses specific to DNFB, because no swelling was observed without prior sensitizations. We found that subcutaneous implantation of placental extract 3 days before sensitization resulted in a significant inhibition of earlobe swelling (Fig. 1A). Titration experiments indicated that no significant inhibition of CHS was achieved when the mice received less than 3 mg placental extract implantation (data not shown), so that 3 mg of extract was given in this study. In histological analyses, DNFB-induced CHS caused severe inflammation in ear tissues showing a typical dermato-pathological appearance of CHS, namely hyperkeratosis, acanthosis, and spongiosis together with marked edema in dermis associated with a massive infiltration of inflammatory cells (Fig. 1B). Consistent with the decreased earlobe swelling, implantation of placental extract alleviated acanthosis, spongiosis, and dermal edema in ear tissues caused by DNFB elicitation. Taken together, our results demonstrated a preventive effect of placental extract in CHS.
Figure 1. Inhibited CHS responses in mice treated with placental extract implantation.
BALB/c mice underwent implantation with 3 mg placental extract (∉) or sham operation (⊄ and Υ) as described in the materials and methods. After 3 days, the mice were sensitized with 0.5% DNFB (∉ and Υ) or vehicle alone (⊄; non-sensitized control), and this procedure was repeated 24 hrs later. Five days after the first sensitization, the mice were challenged with 0.2% DNFB on earlobe, and its thickness was measured everyday. (A) Net increase of earlobe thickness at the indicated time points are shown as the mean +/− SEM (n=5). **P< 0.01, *P< 0.05 between CHS mice treated with placental extract implantation and those with sham operation. (B) Two days after DNFB challenge, earlobe tissues were harvested, processed, and stained with H&E. Representative fields of the tissues from 3 repeated experiments are shown.
Regulation of immune cell distribution and cytokine productions by placental extract administration
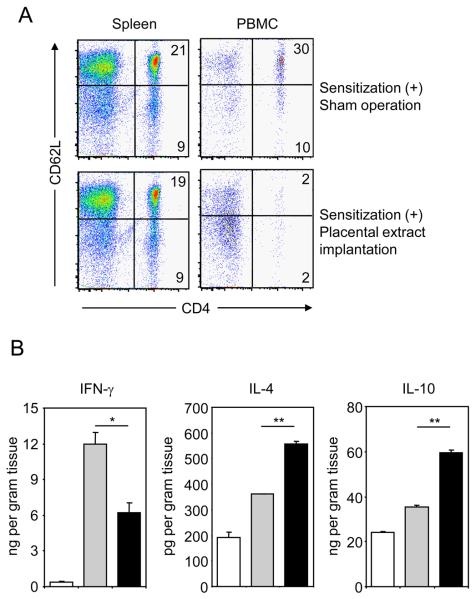
We next assessed immunological changes caused by placental extract implantation in CHS. In spleen (Fig. 2A) and LN (data not shown) of mice treated with placental extract, the number and percentage of immune cells including T, B, NK cells, macrophages and dendritic cells showed no significant differences from sham-operated mice. On the other hand, we found a significant decrease of CD4+ T cells in PBMC of mice implanted with placental extract (Fig. 2A). Among CD4+ T cells, a more striking decrease was detected in CD62L-positive naïve T cells (30% in control vs. 2% in treated mice). No significant changes were observed in CD8+ T cells and other immune cells in PBMC (data not shown). Taken together with the pathological observations, these results suggest that placental extract hampers a potential of CD4+ T cells to locate in the peripheral blood and tissues of CHS. Next, we examined the effects of placental extract implantation on cytokine levels in ear tissues with CHS. Significant inhibition of IFN- and increase of IL-4 and IL-10 was induced by placental extract implantation (Fig. 2B). Collectively, the prevention of CHS by placental extract administration is associated with functional changes of CD4+ T cells, i.e. an inhibited cellular distribution in peripheral tissues and a cytokine profile deviated to Th2-type.
Figure 2. Lymphocyte distribution and cytokine production in mice with CHS after implantation of placental extract.
BALB/c mice underwent implantation with 3 mg placental extract or sham operation. After 3 days, the mice were sensitized with 0.5% DNFB or vehicle alone, and this procedure was repeated 24 hrs later. Five days after the first sensitization, mice were challenged with 0.2% DNFB on the earlobes. (A) Two days after challenge, spleen and PBMC were harvested, stained with anti-CD4 and anti-CD62L mAbs, and analyzed by flow cytometry. The numbers indicate percentages of cells in the corresponding quadrants. (B) Earlobes from mice were harvested and weighed 2 days after DNFB challenge. Cytokine concentration in the supernatants of ground earlobe tissues was measured by ELISA. Cytokine levels per gram tissue of mice without sensitization (open column), with sensitization plus sham operation (gray column), and with sensitization plus placental extract implantation (filled column) is shown as mean +/− SEM (n=3). Representative data from 3 independently repeated experiments are shown. **P< 0.01, *P<0.05.
Preventive effects of placental extract administration in memory CHS responses
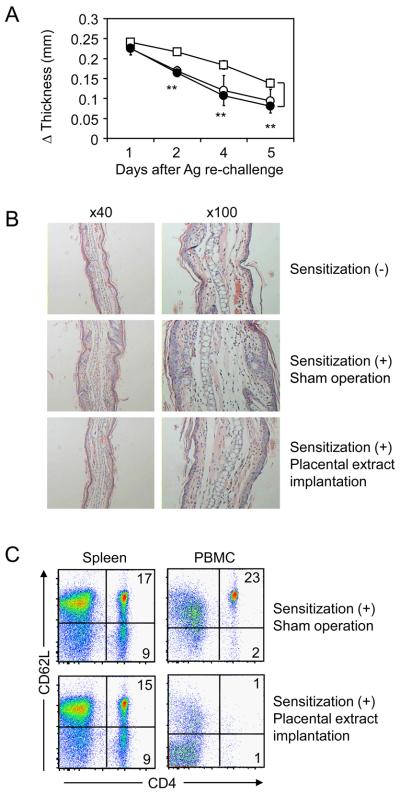
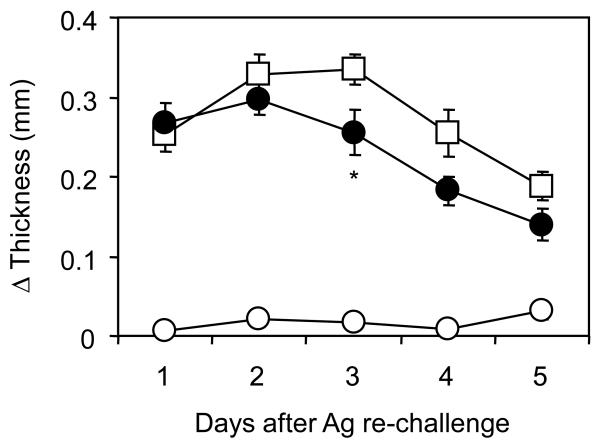
In clinical conditions, repetitive exposures to allergen aggravate symptoms of allergic contact dermatitis due to an enhancement of immunological memory. In order to assess effects of placental extract on memory CHS responses, the mice which had been treated with sensitization and initial elicitation of DNFB were then exposed to a secondary DNFB challenge 3 weeks later. The mice with prior sensitization showed enhanced CHS compared to those without sensitization due to memory responses, while non-sensitized mice still induced a weak CHS because of repeated elicitations (Fig. 3A). Implantation of placental extract in the sensitized mice significantly inhibited memory CHS responses to the level equivalent to those without prior sensitization. Consistently, inflammatory changes in ear tissues were alleviated by placental extract implantation (Fig. 3B). In immunological analysis, placental extract implantation induced a striking decrease of CD62L-positive naive CD4+ T cells in PBMC but not spleen of the mice with memory CHS (Fig. 3C). These changes were concordant with our findings in primary CHS responses (Fig. 2A). Taken together, our results suggest that placental extract administration inhibited memory CHS responses caused by repetitive exposures to allergens.
Figure 3. Preventive effects of placental extract on memory CHS responses.
BALB/c mice underwent implantation with 3 mg placental extract (∉) or sham operation (⊄ and Υ). After 3 days, the mice were sensitized with 0.5% DNFB (∉ and Υ) or vehicle alone (⊄), and this procedure was repeated 24 hrs later. Five days after the first sensitization, the mice were challenged with 0.2% DNFB on earlobe. Then the mice were exposed to a second DNFB challenge 3 weeks after the first challenge, and earlobe thickness was measured thereafter. (A) Net increase of thickness is shown as the mean +/− SEM (n=5). **P< 0.01 between the mice treated with placental extract implantation and those with sham operation. (B) Two days after the second DNFB challenge, earlobe tissues were harvested, processed, and stained with H&E. Representative fields of the tissues are shown. (C) Two days after the second DNFB challenge, spleen and PBMC were harvested from mice, stained with anti-CD4 and anti-CD62L mAbs, and analyzed by flow cytometry. The numbers indicate percentages of cells in the corresponding quadrants. Data are representative from 3 independently repeated experiments.
Therapeutic effects of placental extract administration for CHS in pre-sensitized hosts
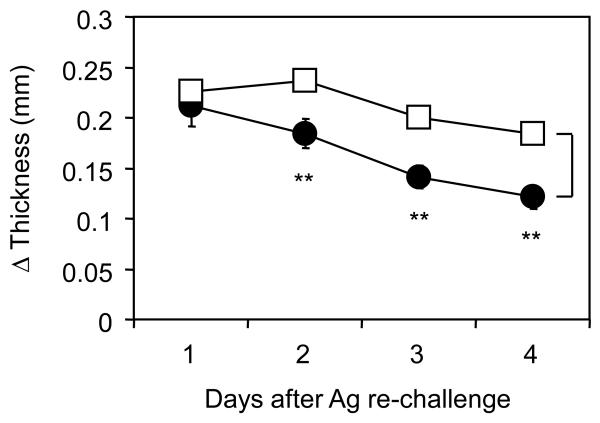
Because most patients of allergic contact dermatitis have been exposed to allergens at their first hospital visit, clinically applicable therapies need to demonstrate its efficacy even when initiated after sensitization. Therefore, we next employed a model in which placental extract was implanted into mice 4 weeks after the initial sensitization of DNFB. In this condition, memory CHS responses induced by the second DNFB challenge were significantly inhibited by placental extract implantation (Fig. 4). Thus, administration of placental extract has a therapeutic potential in memory CHS responses even if the hosts had been exposed to prior sensitizations with allergen.
Figure 4. Therapeutic effects of placental extract on CHS in pre-sensitized mice.
BALB/c mice were sensitized with 0.5% DNFB, and this procedure was repeated 24 hrs later. Five days after the first sensitization, the mice were challenged with 0.2% DNFB on earlobe. Three weeks after the first challenge, the mice underwent implantation with 3 mg placental extract (∉) or sham operation (Υ). Three days after operation, the mice were exposed to a second challenge of DNFB and earlobe thickness was measured thereafter. Net increase of thickness is shown as the mean +/− SEM (n=5). **P< 0.01 between the groups. Representative data from 3 independently repeated experiments are shown.
Treatment of CHS with cyclo-trans-4-L-hydroxyprolyl-L-serine, a dipeptide derived from placental extract
Placental extract contains a variety of biologically active components including, but not limited to, cytokines, chemokines, hormones, nucleotides, small peptides, and fatty acids. While immune-regulatory functions of placental extract are likely dependent on additive and synergistic effects of these components, one of potential candidates is cyclo-trans-4-L-hydroxyprolyl-L-serine, a dipeptide isolated from hydrolysates of placental extract [31, 32]. It has been reported that administration of cyclo-trans-4-L-hydroxyprolyl-L-serine ameliorates inflammation in the liver [33]. Thus, we next examined a potential effect of cyclo-trans-4-L-hydroxyprolyl-L-serine in CHS. Administration of cyclo-trans-4-Lhydroxyprolyl-L-serine ameliorated the severity of CHS (Fig. 5), while its effects were more evident in a late phase of CHS (i.e. after day 3) compared to an early phase (i.e. on day 1 and 2) in which placental extract implantation showed its effect (Fig. 1). These results suggest that cyclo-trans-4-L-hydroxyprolyl-L-serine could be one of components responsible for the therapeutic effects of placental extract in CHS, while other factors may also play an important role.
Figure 5. Therapeutic potential of cyclo-trans-4-L-hydroxyprolyl-L-serine in CHS.
BALB/c mice were injected intraperitoneally with cyclo-trans-4-L-hydroxyprolyl-Lserine (∉) or saline (⊄ and Υ) on day −3, 0, and 3. On day 0, the mice were sensitized with 0.5% DNFB (∉ and Υ) or vehicle alone (⊄; non-sensitized control), and this procedure was repeated 24 hrs later. Five days after the first sensitization, the mice were challenged with 0.2% DNFB on earlobe, and its thickness was measured everyday. Net increase of earlobe thickness at the indicated time points are shown as the mean +/− SEM (n=5). *P< 0.05 between treatment with cyclo-trans-4-L-hydroxyprolyl-L-serine and saline.
Discussion
Effects of placental extract to regulate biological responses and its potential as therapeutic reagents in various diseases have been implicated in numerous studies. Nevertheless, solid evidence demonstrating biological mechanisms of the effects in a well-established experimental system is still lacking. In this study, we demonstrate that administration of placental extract alleviates the severity of CHS, a model mimicking pathological conditions of allergic contact dermatitis. Inhibition of CHS by placental extract is associated with an impaired distribution of CD4+ T lymphocytes to peripheral organs and a relative deviation of cytokine profile toward Th2-type responses. Memory CHS responses are also inhibited by placental extract administration prior to Ag re-challenge, suggesting its therapeutic potential in actual clinical conditions of contact dermatitis in which symptoms are triggered and worsened by repetitive Ag exposures. Finally, administration of placental extract-derived cyclo-trans-4-L-hydroxyprolyl-Lserine also alleviated CHS severity, suggesting its potential role in the inhibitory effects of placental extract, while it is conceivable that other components in placental extract and synergy of multiple factors play an important role in the regulation of CHS in our model.
Inflammatory cytokines represent pathological factors essential for CHS [37]. In a model using DNFB as a hapten, Th1-type cytokines produced by T lymphocytes plays a crucial role [34, 38]. On the other hand, Th2-type cytokines including IL-10 produced by regulatory T cells inhibit CHS via downregulation of MHC and co-stimulatory molecules on APC and reciprocal suppression of Th1 cells [39-41]. Consistent with this mechanism, our study demonstrates that administration of placental extract alleviates CHS severity concomitantly with a decrease of IFN- and increase of IL-4/IL-10 production. In this context, it should be noted that placenta is a potent IL-10 inducer, as demonstrated by studies that placental trophoblasts directly produce IL-10 and that placenta-derived factors indirectly stimulate IL-10 production from immune cells [42, 43]. In addition, B7-H1, an inhibitory co-signal molecule which was cloned from placenta and is essential for fetal-maternal tolerance, stimulates IL-10 production from T cells and suppresses inflammatory responses including CHS [35, 44, 45]. Taken together, it is possible that placenta-derived factors, such as B7-H1, render a cytokine environment suppressive and therapeutic for CHS in our model, while its causative role will be further addressed by experiments using mice deficient of these molecules or reagents to abrogate its functions. Another potential cytokine targeted by placental extract would be IL-17, as recent studies suggested that Th17-type responses play a pathological role of in CHS [46].
In the mice treated with placental extract, a profound decrease of CD4+ T cells in PBMC and reduced numbers of infiltrating lymphocytes in the ear tissues were observed. These findings can be explained by two potential mechanisms; 1) an impaired ability of CD4+ T cells in exiting from secondary lymphoid organs and emigrating to peripheral organs, and 2) an impaired survival of CD4+ T cells selectively in the peripheral organs. These potential mechanisms are not mutually exclusive. The first mechanism is reminiscent of the immunoregulatory effects of FTY720, an agonist of sphingosine-1-phosphate (S1P) receptor [47]. It should be noted that placental sphingolipids including S1P are crucial regulators for biological functions of placenta [48], and that FTY720 has been shown to alleviate CHS severity by interfering with T lymphocyte migration to PBMC [49]. As for the second mechanism, it has been reported that placenta-derived factors including Fas-ligand and TRAIL induce apoptosis of immune cells, thereby contributing to fetal-maternal tolerance [50]. In this scenario, however, it remains unclear how placental extract induces reduction of CD4+ T cells selectively in the non-lymphoid organs.
Administration of placental extract demonstrated therapeutic efficacy in CHS caused by repetitive Ag challenges. This result suggests that CHS induced by memory T cells as well as naïve T cells can be inhibited by placenta-derived factors, probably due to a change of cytokine milieu and migratory property of lymphocytes as discussed above. Taken together, our current studies provided experimental observations which indicate a therapeutic potential of placental extract in contact dermatitis and elucidated its immunological mechanisms.
Acknowledgement
We thank Drs. Ryusuke Omiya and Atsuo Kuramasu for helpful discussion, and Yingjia Liu for technical assistance. This work was supported in part by R01HL088954 to K.T.
Abbreviations
- CHS
contact hypersensitivity
- Ag
antigen
- Ab
antibody
- LN
lymph nodes
- DNFB
dinitrofluorobenzene
- PBMC
peripheral blood mononuclear cells
- S1P
sphingosine-1-phosphate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Tai-ichi Kaku holds stock of Japan Bio Products Co., Ltd.
References
- 1.Wolf HK, Zarnegar R, Oliver L, et al. Hepatocyte growth factor in human placenta and trophoblastic disease. Am J Pathol. 1991;138:1035–43. [PMC free article] [PubMed] [Google Scholar]
- 2.Qu J, Thomas K. Regulation of inhibin secretion in human placental cell culture by epidermal growth factor, transforming growth factors, and activin. J Clin Endocrinol Metab. 1993;77:925–31. doi: 10.1210/jcem.77.4.8408467. [DOI] [PubMed] [Google Scholar]
- 3.Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–5. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 4.Sur TK, Biswas TK, Ali L, et al. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol Sin. 2003;24:187–92. [PubMed] [Google Scholar]
- 5.Liu KX, Kato Y, Kaku T, et al. Human placental extract stimulates liver regeneration in rats. Biol Pharm Bull. 1998;21:44–9. doi: 10.1248/bpb.21.44. [DOI] [PubMed] [Google Scholar]
- 6.Kong MH, Lee EJ, Lee SY, et al. Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause. 2008;15:296–303. doi: 10.1097/gme.0b013e3181405b74. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee KK, Bishayee A, Chatterjee M. Effects of human placental extract on brain monoamines and monoamine oxidase activity in rats. Tohoku J Exp Med. 1995;176:17–24. doi: 10.1620/tjem.176.17. [DOI] [PubMed] [Google Scholar]
- 8.Pal P, Mallick S, Mandal SK, et al. A human placental extract: in vivo and in vitro assessments of its melanocyte growth and pigment-inducing activities. Int J Dermatol. 2002;41:760–7. doi: 10.1046/j.1365-4362.2002.01524.x. [DOI] [PubMed] [Google Scholar]
- 9.Tiwary SK, Shukla D, Tripathi AK, et al. Effect of placental-extract gel and cream on non-healing wounds. J Wound Care. 2006;15:325–8. doi: 10.12968/jowc.2006.15.7.26937. [DOI] [PubMed] [Google Scholar]
- 10.Frolik CA, Dart LL, Meyers CA, et al. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983;80:3676–80. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann GE, Scott RT, Jr., Bergh PA, et al. Immunohistochemical localization of epidermal growth factor in human endometrium, decidua, and placenta. J Clin Endocrinol Metab. 1991;73:882–7. doi: 10.1210/jcem-73-4-882. [DOI] [PubMed] [Google Scholar]
- 12.Chen CF, Kurachi H, Fujita Y, et al. Changes in epidermal growth factor receptor and its messenger ribonucleic acid levels in human placenta and isolated trophoblast cells during pregnancy. J Clin Endocrinol Metab. 1988;67:1171–7. doi: 10.1210/jcem-67-6-1171. [DOI] [PubMed] [Google Scholar]
- 13.Filla MS, Zhang CX, Kaul KL. A potential transforming growth factor alpha/epidermal growth factor receptor autocrine circuit in placental cytotrophoblasts. Cell Growth Differ. 1993;4:387–93. [PubMed] [Google Scholar]
- 14.Mitchell EJ, Fitz-Gibbon L, O'Connor-McCourt MD. Subtypes of betaglycan and of type I and type II transforming growth factor-beta (TGF-beta) receptors with different affinities for TGF-beta 1 and TGF-beta 2 are exhibited by human placental trophoblast cells. J Cell Physiol. 1992;150:334–43. doi: 10.1002/jcp.1041500217. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Yang T, Wang C, et al. Laennec protects murine from concanavalin A-induced liver injury through inhibition of inflammatory reactions and hepatocyte apoptosis. Biol Pharm Bull. 2008;31:2040–4. doi: 10.1248/bpb.31.2040. [DOI] [PubMed] [Google Scholar]
- 16.Degenne D, Canepa S, Horowitz R, et al. Effect of human syncytiotrophoblast extract on in vitro proliferative responses. Am J Reprod Immunol Microbiol. 1985;8:20–6. doi: 10.1111/j.1600-0897.1985.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 17.Arkwright PD, Rademacher TW, Boutignon F, et al. Suppression of allogeneic reactivity in vitro by the syncytiotrophoblast membrane glycocalyx of the human term placenta is carbohydrate dependent. Glycobiology. 1994;4:39–47. doi: 10.1093/glycob/4.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Voisin JE, Kinsky RG, Voisin GA. Maternal alloimmune reactions towards the murine conceptus and graft-versus-host reaction (GVHR). II. Inhibition of priming by placental extracts. J Reprod Immunol. 1986;9:85–94. doi: 10.1016/0165-0378(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 19.Bobe P, Doric M, Kinsky RG, et al. Modulation of mouse anti-SRBC antibody response by placental extracts. Cell Immunol. 1984;89:355–64. doi: 10.1016/0008-8749(84)90337-x. [DOI] [PubMed] [Google Scholar]
- 20.Garg R, Zahra F, Chandra JA, et al. A comparative study of injection placentrex and conventional therapy in treatment of pelvic inflammatory disease. J Indian Med Assoc. 2008;106:463–467. [PubMed] [Google Scholar]
- 21.Kang SS, Woo SS, Im J, et al. Human placenta promotes IL-8 expression through activation of JNK/SAPK and transcription factors NF-kappaB and AP-1 in PMA-differentiated THP-1 cells. Int Immunopharmacol. 2007;7:1488–95. doi: 10.1016/j.intimp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Gu Y, Philibert L, et al. Neutrophil activation induced by placental factors in normal and pre-eclamptic pregnancies in vitro. Placenta. 2001;22:560–5. doi: 10.1053/plac.2001.0691. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty D, Basu JM, Sen P, et al. Human placental extract offers protection against experimental visceral leishmaniasis: a pilot study for a phase-I clinical trial. Ann Trop Med Parasitol. 2008;102:21–38. doi: 10.1179/136485908X252133. [DOI] [PubMed] [Google Scholar]
- 24.Skoet R, Zachariae R, Agner T. Contact dermatitis and quality of life: a structured review of the literature. Br J Dermatol. 2003;149:452–6. doi: 10.1046/j.1365-2133.2003.05601.x. [DOI] [PubMed] [Google Scholar]
- 25.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Unger M, Tuvel B, et al. Contact hypersensitivity: the mechanism of immune responses and T cell balance. J Interferon Cytokine Res. 2002;22:407–12. doi: 10.1089/10799900252952181. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Mezard P, Berard F, Dubois B, et al. The role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis. Eur J Dermatol. 2004;14:131–8. [PubMed] [Google Scholar]
- 28.Wang B, Feliciani C, Freed I, et al. Insights into molecular mechanisms of contact hypersensitivity gained from gene knockout studies. J Leukoc Biol. 2001;70:185–91. [PubMed] [Google Scholar]
- 29.O'Keefe EJ, Chiu ML. Stimulation of thymidine incorporation in keratinocytes by insulin, epidermal growth factor, and placental extract: comparison with cell number to assess growth. J Invest Dermatol. 1988;90:2–7. doi: 10.1111/1523-1747.ep12462409. [DOI] [PubMed] [Google Scholar]
- 30.Cho HR, Ryou JH, Lee JW, et al. The effects of placental extract on fibroblast proliferation. J Cosmet Sci. 2008;59:195–202. [PubMed] [Google Scholar]
- 31.Liu KX, Kato Y, Kaku TI, et al. Hydroxyprolylserine derivatives JBP923 and JBP485 exhibit the antihepatitis activities after gastrointestinal absorption in rats. J Pharmacol Exp Ther. 2000;294:510–5. [PubMed] [Google Scholar]
- 32.Wu J, Wang C, Liu Q, et al. Protective effect of JBP485 on concanavalin A-induced hepatocyte toxicity in primary cultured rat hepatocytes. Eur J Pharmacol. 2008;589:299–305. doi: 10.1016/j.ejphar.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 33.Yang T, Wu J, Wang C, et al. Protective effect of JBP485 on concanavalin A-induced liver injury in mice. J Pharm Pharmacol. 2009;61:767–74. doi: 10.1211/jpp.61.06.0009. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Fujisawa H, Zhuang L, et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol. 2000;165:6783–90. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- 35.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson TA, Dube P, Griffith TS. Regulation of contact hypersensitivity by interleukin 10. J Exp Med. 1994;179:1597–604. doi: 10.1084/jem.179.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gober MD, Gaspari AA. Allergic contact dermatitis. Curr Dir Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- 38.Ishizaki K, Yamada A, Yoh K, et al. Th1 and type 1 cytotoxic T cells dominate responses in T-bet overexpression transgenic mice that develop contact dermatitis. J Immunol. 2007;178:605–12. doi: 10.4049/jimmunol.178.1.605. [DOI] [PubMed] [Google Scholar]
- 39.Kondo S, McKenzie RC, Sauder DN. Interleukin-10 inhibits the elicitation phase of allergic contact hypersensitivity. J Invest Dermatol. 1994;103:811–4. doi: 10.1111/1523-1747.ep12413470. [DOI] [PubMed] [Google Scholar]
- 40.Simkin GO, Tao JS, Levy JG, et al. IL-10 contributes to the inhibition of contact hypersensitivity in mice treated with photodynamic therapy. J Immunol. 2000;164:2457–62. doi: 10.4049/jimmunol.164.5.2457. [DOI] [PubMed] [Google Scholar]
- 41.Ring S, Schafer SC, Mahnke K, et al. CD4+ CD25+ regulatory T cells suppress contact hypersensitivity reactions by blocking influx of effector T cells into inflamed tissue. Eur J Immunol. 2006;36:2981–92. doi: 10.1002/eji.200636207. [DOI] [PubMed] [Google Scholar]
- 42.Roth I, Corry DB, Locksley RM, et al. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–48. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahum R, Brenner O, Zahalka MA, et al. Blocking of the placental immune-modulatory ferritin activates Th1 type cytokines and affects placenta development, fetal growth and the pregnancy outcome. Hum Reprod. 2004;19:715–22. doi: 10.1093/humrep/deh099. [DOI] [PubMed] [Google Scholar]
- 44.Tsushima F, Iwai H, Otsuki N, et al. Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur J Immunol. 2003;33:2773–82. doi: 10.1002/eji.200324084. [DOI] [PubMed] [Google Scholar]
- 45.Guleria I, Khosroshahi A, Ansari MJ, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–7. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J Immunol. 2009;183:1463–70. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 48.Mizugishi K, Li C, Olivera A, et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashima D, Kabashima K, Sakabe J, et al. Impaired initiation of contact hypersensitivity by FTY720. J Invest Dermatol. 2008;128:2833–41. doi: 10.1038/jid.2008.174. [DOI] [PubMed] [Google Scholar]
- 50.Riley JK. Trophoblast immune receptors in maternal-fetal tolerance. Immunol Invest. 2008;37:395–426. doi: 10.1080/08820130802206066. [DOI] [PubMed] [Google Scholar]