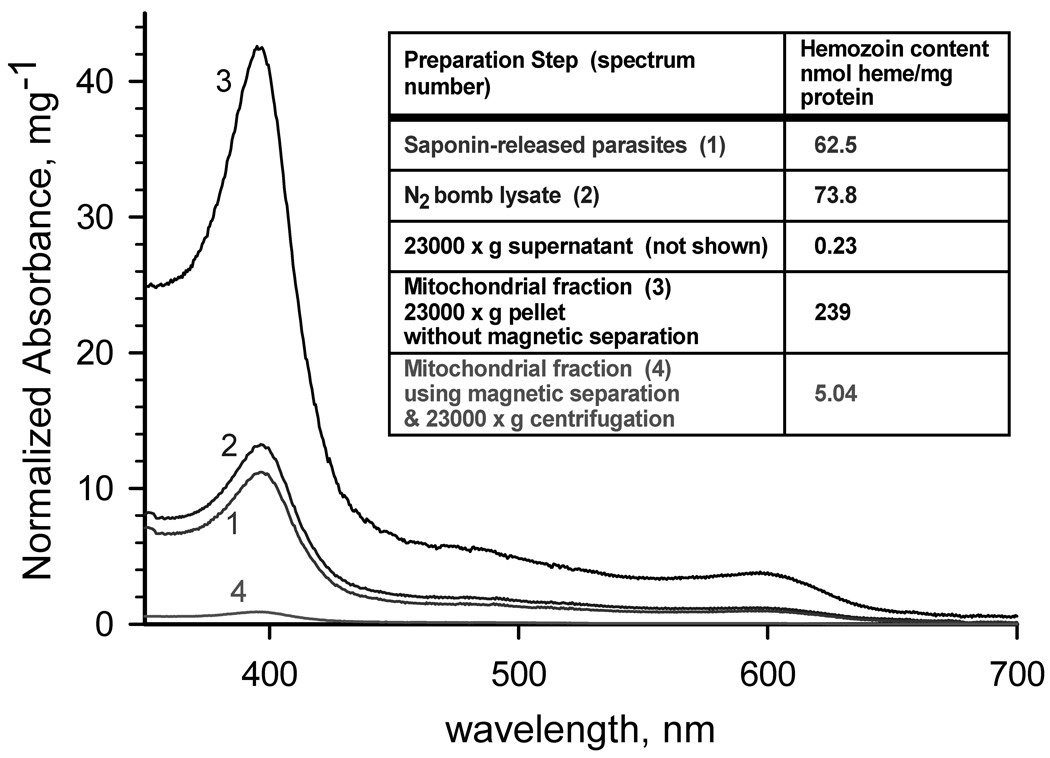
Fig. 1. Hemozoin content of fractions of a mitochondrial preparation.
Spectra of hemozoin extracted from (1) saponin-released parasites, (2) parasite lysate produced by N2 cavitation, (3) 23000g pellet without a magnetic separation step, and (4) 23000g pellet obtained after magnetic separation using MACS. Inset: Calculated hemozoin contents. Parasite mitochondria were isolated from P. falciparum cultures that were synchronized at least twice by treatment with alanine [11], expanded and harvested at 8–15% parasitemia in the mid to late trophozoite stage. Parasitized erythrocytes were harvested by centrifugation, and lysed at 37°C with 0.05% (w/v) saponin in lysis buffer (120 mM KCl, 20 mM NaCl, 20 mM glucose; 6 mM (HEPES), 6 mM MOPS, 1 mM MgCl2, 0.1 mM EGTA, pH 7.0). After washing 3 times with lysis buffer and once with mitochondrial isolation buffer (225 mM mannitol, 75 mM sucrose, 4.3 mM MgCl2, 0.25 mM EGTA, 10 mM HEPES [Tris], 5 mM HEPES [KOH]; pH 7.4) containing 5 mM glucose, the parasites were pressurized with compressed nitrogen in a 4639 Cell Disruption Bomb (N2 bomb) (Parr, USA) at 1000 psi for 20 min at 4°C in reduced-oxygen mitochondrial isolation buffer containing 5 mM glucose and mitochondrial substrates (2.5 mM succinate, 5 mM D,L-malate, 2 mM α-glycerophosphate, and 1 mM dihydroorotate) in the presence of 1 mM phenylmethylsulfonyl fluoride and 1 µl per ml fungal protease inhibitor cocktail (Sigma-Aldrich, Inc., St. Louis, MO, USA). Cavitation was performed by slow drop-wise release of the parasite suspension through the valve of the N2 bomb. The 1000 psi pressure in the N2 bomb was reestablished whenever the release caused a drop of more than 100 psi. Upon completion of the disruption, another aliquot of protease inhibitors was added, and the unbroken cells and cell debris were removed by centrifugation at 900×g for 6 min at 4°C. The low speed supernatant was passed slowly (~0.2 ml/min) through a MACS CS column prewashed with mitochondrial isolation buffer in a Vario MACS magnetic separation apparatus (Miltenyi Biotec, Auburn, CA, USA) to remove hemozoin. The mitochondria were recovered as a pellet by centrifugation at 23000×g for 20 min at 4°C. The supernatant was removed as completely as possible, and the pellet was suspended in a minimal volume of mitochondrial isolation buffer containing 0.75 mM succinate (and/or 0.5 mM dihydroorotate, if DHODH activity is to be measured) and used for enzymatic assay or stored at −80°C. To determine hemozoin content, protein and lipids were removed from each sample aliquot using 2 washes with 2 % sodium dodecyl sulfate, and the hemozoin collected by centrifugation. The hemozoin pellet was depolymerized as described by Sullivan et al [12]. The solution was neutralized with HEPES, and the heme concentration in the sample was determined spectroscopically (ε400nm = 100,000 M−1cm−1).