Abstract
Burkholderia cenocepacia AU1054 is an opportunistic pathogen isolated from the blood of a person with cystic fibrosis. AU1054 is a multihost pathogen causing rapid pathogenicity to Caenorhabditis elegans nematodes. Within 24 h, AU1054 causes greater than 50% mortality, reduced growth, emaciated body, distended intestinal lumen, rectal swelling, and prolific infection of the nematode intestine. To determine virulence mechanisms, 3,000 transposon mutants were screened for attenuated virulence in nematodes. Fourteen virulence-attenuated mutants were isolated, and the mutant genes were identified. These genes included paaA, previously identified as being required for full virulence of B. cenocepacia K56-2. Six mutants were restored in virulence by complementation with their respective wild-type gene. One of these contained an insertion in gspJ, predicted to encode a pseudopilin component of the type 2 secretion system (T2SS). Nematodes infected with AU1054 gspJ had fewer bacteria present in the intestine than those infected with the wild type but still showed rectal swelling. The gspJ mutant was also defective in pathogenicity to onion and in degradation of polygalacturonic acid and casein. This result differs from previous studies where no or little role was found for T2SS in Burkholderia virulence, although virulence factors such as zinc metalloproteases and polygalacturonase are known to be secreted by the T2SS. This study highlights strain specific differences in B. cenocepacia virulence mechanisms important for understanding what enables environmental microbes to function as opportunistic pathogens.
The betaproteobacterium Burkholderia cenocepacia is a member of the Burkholderia cepacia complex (BCC), now consisting of 17 classified species (72). Members of the BCC are ubiquitous in the environment, metabolically diverse, and beneficial or pathogenic to a variety of organisms and have large and dynamic multireplicon genomes (11, 48). Originally described as a pathogen to onion plants (9), members of the BCC have emerged as opportunistic pathogens of serious concern to persons with cystic fibrosis (CF) or chronic granulomatous diseases (26, 29, 36). Although Pseudomonas aeruginosa is more commonly isolated from CF infections, infections with BCC also occur and are a serious concern because of their inherent multidrug resistance and correlation with the severe loss of lung function, sepsis, and fatality referred to as cepacia syndrome (36). Infections of CF patients arise from patient-to-patient transmission of epidemic clones or sporadically from a presumed environmental source (29). Epidemic strains of B. cenocepacia and B. multivorans are correlated with the incidence of cepacia syndrome (49, 75). The PHDC epidemic clone strain, B. cenocepacia AU1054 (called AU1054 hereafter), is a multihost pathogen with high virulence to the nematode Caenorhabditis elegans and to onions (13, 44, 64).
The C. elegans model for the study of bacterial pathogenicity was originally developed for P. aeruginosa (20, 69), which causes nematode mortality by two mechanisms termed fast killing (within hours, toxin mediated) (46, 69) and slow killing (in days, infection mediated) (69). Virulence factors required for C. elegans killing are sometimes also involved in multihost virulence (e.g., plant and animal) (45). The C. elegans model has now been established for the study of virulence of a large variety of pathogens including BCC (10, 19, 41). AU1054 is highly pathogenic to C. elegans killing nematodes fast on minimal medium where P. aeruginosa kills slowly by an unknown mechanism (64).
Type 2 secretion systems (T2SSs) are required for the secretion of many toxins and enzymes that contribute to virulence, notably exotoxin A and cholera toxin produced by P. aeruginosa and Vibrio cholerae, respectively (23, 62). Previous studies did not detect a significant defect in virulence of B. pseudomallei, B. cenocepacia, and B. vietnamiensis strains mutant in T2SS, although the T2SS is required for B. gladioli pathogenicity to mushrooms (14, 21, 24, 41). However, expression of the T2SS genes was found to be induced in synthetic CF sputum medium relative to soil extract by strains AU1054 and J2315; although the latter was unexpectedly found to have a 110-bp deletion in gspL, known to be required for T2SS function (34, 73, 74). These contradictory results suggest that it is possible that the T2SS is important for BCC to function as opportunistic pathogens.
We describe here the application of the C. elegans model for the study of multihost pathogenicity of the CF epidemic B. cenocepacia strain AU1054. Our goal was to identify genes required for AU1054 pathogenicity using the C. elegans model and to determine the role of these genes in pathogenicity to multiple hosts.
MATERIALS AND METHODS
Strains and media.
The bacterial and nematode strains and plasmids used in the present study are listed in Table 1. Unless otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Strain AU1054 was chosen for this study because it was isolated from the blood of a person with cystic fibrosis, is a member of the PHDC epidemic clonal lineage, is highly virulent to C. elegans nematodes and onions (64), produces high antifungal activity against Rhizoctonia solani AG4, and hence was an ideal candidate for analysis of multihost pathogenicity. AU1054 was cultured on LB medium modified to contain 5 g of NaCl/liter and amended with 1.5% agar (Becton Dickinson, Franklin Lakes, NJ), 150 μg of chloramphenicol/ml, and 300 μg of gentamicin/ml when required. For Escherichia coli, LB medium was amended with 100 μg of ampicillin/ml, 120 μg of chloramphenicol/ml, 10 μg of gentamicin/ml, 30 μg of kanamycin/ml, 20 μg of streptomycin/ml, and 300 μg of diaminopimelic acid (DAP)/ml when required. The cultures were grown aerobically at 37°C.
TABLE 1.
Strains and plasmids used in the study
| Strain or plasmid | Relevance or genotypea | Source or reference |
|---|---|---|
| Strains | ||
| Nematode | ||
| C. elegans SS104 | C. elegans strain SS104 [glp-4(bn2)I], ts sterile at 25°C | Caenorhabditis Genetics Center, University of Minnesota |
| Bacteria | ||
| B. cenocepacia AU1054 | CF clinical isolate, U.S. PHDC epidemic clone | J. J. LiPuma, U.S. Burkholderia cepacia Research Laboratory and Repository, University of Michigan |
| P. aeruginosa PA14 | Control CF pathogen | 69 |
| E. coli OP50 | Nematode food source | 8 |
| E. coli BW29427 | dap auxotroph, tra pir | K. A. Datsenko and B. L. Wanner, Purdue University |
| E. coli EC100 pir-116 | π protein for replication of oriR6Kγ | Epicentre Biotechnologies |
| Plasmids | ||
| pBBR1MCS | Broad-host-range cloning vector; Cmr | 42 |
| pURR25 | Mini Tn7KsGFP, GFP driven by Plac (PA1/04/03) promoter, mobilizable oriTIncPα, suicide oriRR6kγ; Apr (bla) | D. Lies and D. Newman, Caltech |
| pURE10 | Mini-HimarGm transposon, C9 transposase, suicide oriR6Kγ; Gmr Apr | D. Lies and D. Newman, Caltech |
| pUX-BF13 | Tn7 transposase genes tnsABCDE, mobilizable oriTIncPα, suicide oriRR6kγ; Apr (bla) | 4 |
| pCS238 | Source of flmAB-ccdAB toxin-antitoxin cassette | 12 |
| pTCV2 | pBBR1MCS flmAB-ccdAB (toxin-antitoxin cassette); Cmr | This study |
| pTCV3 | pTCV2 Ptac::GFP; Cmr | This study |
| pbnvR | pTCV2 bnvR | This study |
| pnhaX | pTCV2 nhaX | This study |
| pargG | pTCV2 argG | This study |
| paroA | pTCV2 aroA | This study |
| pgspJ | pTCV2 gspJ | This study |
| ppurF | pTCV2 purF | This study |
Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Apr, ampicillin resistance.
GFP labeling.
Green fluorescent protein (GFP)-labeled strains of E. coli OP50, P. aeruginosa PA14, and B. cenocepacia AU1054 were made by inserting a mini-Tn7 transposon containing Plac::GFP into the bacterial strains by triparental mating as described previously (17). Briefly, donor strain BW29427 (K. A. Datsenko and B. L. Wanner, unpublished data) containing either pURR25 (plasmid containing the miniTn7KSGFP) or pUX-BF13 (encoding Tn7 transposase) were grown and mated with the AU1054 cells by triparental mating on LB agar containing DAP. GFP-labeled recipient cells were selected by resistance to kanamycin and streptomycin (BW29427 requires DAP to grow) and screened for GFP fluorescence by using a MZ16F stereo-fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Molecular biological techniques.
Standard molecular techniques were used as previously described (60). Enzymes used were obtained from New England Biolabs (Ipswich, MA) or Invitrogen (Carlsbad, CA) unless otherwise indicated. Genomic DNA was purified by using a DNeasy blood and tissue kit (Qiagen, Valencia, CA) and plasmid DNA was purified by using a Mini or Midi plasmid kit (Qiagen).
Transposon mutagenesis.
GFP-labeled AU1054 was mutagenized by the hyperactive mariner HimarGm transposon present on the suicide plasmid pURE10. pURE10 was purified from EC100 pir-116 (Epicentre, Madison, WI) prior to electroporation into AU1054. Electrocompetent AU1054 cells were prepared from 1 ml of a mid-exponential-phase culture that was washed and concentrated using sterile 10% (wt/vol) glycerol solution. AU1054 HimarGm mutants were selected on LB agar containing gentamicin. Three hundred mutants were screened in each of 10 independent mutagenesis experiments.
Screening for virulence attenuated mutants by nematode pathogenesis assays.
C. elegans strain SS104 [glp-4(bn2)I], which is sterile at 25°C but fertile at 15°C, was used for nematode pathogenicity assays on nematode growth medium (NGM), P. aeruginosa PA14 was used as a positive control, and E. coli OP50 served as the negative control. The nematodes were maintained on E. coli OP50 lawns on NGM plates at 15°C and synchronized by collecting eggs from the gravid nematodes using bleach-alkali solution. The eggs were transferred to OP50 lawns and allowed to develop to early L4 stage (25°C for 36 to 38 h) used for the assays.
Bacterial strains were grown in LB medium in 96-well microtiter plates (Corning, Inc., Corning, NY) for 14 to 16 h at 37°C, with shaking at 250 rpm. Then, 75 μl of these liquid cultures was spread on 60-by-15-mm petri plates containing NGM, followed by incubation at 37°C for 16 to 18 h to get confluent bacterial lawns of all strains on the NGM assay plates. A total of 40 to 60 early L4-stage C. elegans nematodes were added to the plates containing bacterial lawns, and the number of surviving nematodes was determined after 3 days as those moving or responding to touch. Putative mutants attenuated in C. elegans pathogenicity were verified in three independent experiments consisting of three replicates each.
The mean of 3-day nematode survival data from mutant screens and complementation experiments was compared by using Student t test to determine the statistical significance (P = 0.05). Further survival analysis of nematodes on lawns of AU1054 mutant and complemented strains was performed by counting the number of surviving nematodes daily for 7 days and then analyzing the data by using Kaplan-Meier statistical method. The log-rank test was used to compare the survival differences for statistical significance (P < 0.05) using software GraphPad Prism (version 5; GraphPad Software, Inc., La Jolla, CA). Survival experiments were performed in triplicate and repeated at least twice.
Determination of the DNA sequence flanking the HimarGm transposons in the virulence-attenuated mutants.
The HimarGm transposon contains an R6Kγ ori, which is functional in EC100 pir-116 (Epicentre), allowing the transposon and flanking DNA to be cloned by marker rescue (17). Briefly, 5 to 10 μg of mutant genomic DNA was digested with SphI that cuts in DNA flanking the transposon, ligated, and concentrated before transformation into EC100 pir-116. Transformants containing the HimarGm were selected on LB+Gm plates. Plasmids purified from three clones of each mutant were digested with SacI and SphI to determine the presence of the HimarGm (835-bp fragment) and size of the retrieved plasmid, respectively. The DNA sequence of unique plasmids was determined by sequencing using the primers MarOUT and GMRFor (Table 2), performed at the Research Technology Support Facility at Michigan State University. The exact physical location of the HimarGm was determined by BLASTn analysis of flanking DNA to the AU1054 genome present on one of several genome databases (e.g., the Burkholderia Genome Database [www.burkholderia.com]) (2).
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide or complementation primer | Sequence (5′-3′)a | Restriction enzyme |
|---|---|---|
| Oligonucleotides | ||
| TetR-Forward | GATCCTGCAGGCGGCCGCCCACCGCGGTGGGATATCATGAAATCTAACAATGCGCTC | PstI |
| TetR-Reverse | GATCCTGCAGTGCACCGCGACGCAACGC | PstI |
| MarOUT | CCGGGGACTTATCAGCCAACC | |
| GMRFor | CGGTAAATTGTCACAACGCC | |
| GFPFor | GAACTAGTCTCGAGAAAATTTATCAAAAAGAGTGTTGACTT | SpeI |
| GFPRev | GAACTAGTTTATTTGTATAGTTCATCCATGCCATGTGTAATC | SpeI |
| Lower flm | TTCGTCTAGACCTGGCAGTCTGGTTGTTCAT | XbaI |
| Lower ccd | CCGATCTAGACTGCAGACTGGCTGTGTATAAC | XbaI |
| Complementation primers | ||
| B-cen_0002 C-F | GCATGGTACCACAATCCAGATCGGCAACCT | KpnI |
| B-cen_0002 C-R | CTAGACTAGTTCATGCTTTCGGTGATTTCC | SpeI |
| B-cen_0015 C-F | GCATGGTACCGGCGAACAGAGTTTCGACTG | KpnI |
| B-cen_0015 C-R | CTAGACTAGTGATCACGCGCTGGTTCTC | SpeI |
| B-cen_0566 C-F | GCATGGTACCGACGAACTCGACGGCTACAC | KpnI |
| B-cen_0566 C-R | GCTAACTAGTCTGTCGAGCAGGTGAAAGC | SpeI |
| B-cen_3021 C-F | GCATGGTACCGGGACTGACGATTCGCATAC | KpnI |
| B-cen_3021 C-R | CTGAACTAGTCTTCGAGTACCTCGCGTTCT | SpeI |
| B-cen_4402 C-F | GCATGGTACCAACTGCCCAAACAGGATTTC | KpnI |
| B-cen_4402 C-R | CTAGACTAGTAAAACAAAAAGCCCGCTATG | SpeI |
| B-cen_4651 C-F | GCATGGTACCGTTCTCCAGCCGGCTCAC | KpnI |
| B-cen_4651 C-R | CTAGACTAGTATTCGCAGCTTGCCATGA | SpeI |
Restriction enzyme sites are indicated in boldface.
Complementation.
We improved a previously described broad host range vector pBBR1MCS (42) for the complementation assays for better stability in Burkholderia by adding two toxin-antitoxin cassettes. The flmAB-ccdAB toxin-antitoxin cassette was amplified from pCS238 by using primers with XbaI sites (Table 2). The plasmid pBBR1MCS and the flmAB-ccdAB toxin-antitoxin cassette were digested with XbaI and ligated to create the pBBR1MCS flmAB-ccdAB, which was named pTCV2. The reporter cassette Plac::GFP was PCR amplified from the plasmid pURR25 by using the primers GFPFor and GFPRev with SpeI restriction sites added to the termini (Table 2), digested with SpeI, and cloned into the SpeI site in the multiple cloning site (MCS) of pTCV2 to create pTCV3, which was used to check the stability of this plasmid vector in Burkholderia prior to complementation assays.
Vector pTCV2 was used for the complementation assays. The genes to be complemented were amplified from wild-type AU1054 by using Platinum Taq DNA polymerase high fidelity (Invitrogen) and cloned directionally downstream of the T3 promoter of pTCV2 after digestion with the appropriate restriction enzyme(s) (Table 2). All of the constructs made as described above were first transformed into E. coli strain DH5α and then transformed into the respective AU1054 mutant strains as described above.
C. elegans pathology.
To observe bacterial infection- and pathogenesis-related symptoms, the nematodes were inoculated onto GFP-labeled mutants, wild-type AU1054, P. aeruginosa PA14, and E. coli OP50 lawns as described above. After 24 to 48 h, the nematodes were washed off the lawns in 1.5 ml of Ringer's (100 mM NaCl, 1.8 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES [pH 6.9]) and washed again prior to imaging. Nematodes were observed by fluorescent and differential interference contrast microscopy using a Leica DM5000 compound microscope (Leica) equipped with an X-cite 120 fluorescence illuminator (EXFO, Quebec, Canada), a Spot Pursuit charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI), and a GFP filter set (Leica).
Onion pathogenesis assays.
Onion pathogenicity assays were performed as described previously (64). Briefly, quartered onion scales were wounded with a sterile pipette tip and then inoculated with 107 bacterial cells in 5 μl, followed by incubation at 37°C for 48 h. The degree of maceration was then determined by probing with a sterile toothpick, and the area of water-soaking symptoms was measured with an absolute digimatic caliper (Mitutoyo Corp., Aurora, IL). Area measurement treatment means were separated by using a two-tailed t test (P < 0.05). Uninoculated King's B (KB) media or B. cepacia ATCC 25416 served as negative and positive controls, respectively. Experiments were performed at least four times in triplicate.
Polygalacturonic acid degradation assays.
The polygalacturonase enzyme activity of AU1054 wild type and transposon mutants was examined on Hildebrand's polygalacturonic acid gel medium at pH 4.5 to 4.7 (31). One microliter of an overnight-grown bacterial culture in KB medium was spotted in quadruplicate onto the gel surface of a Hildebrand medium plate, followed by incubation at 30°C for 48 h. The degradation of polygalacturonic acid was evaluated by measuring the diameter of the zone of degraded pectin substrate. Uninoculated KB and Burkholderia ambifaria AMMD served as negative controls. Three independent experiments with four replications were performed.
Fungal antibiosis assays.
Antifungal activity was determined against Rhizoctonia solani as described previously (64). Briefly, 2 μl of culture grown overnight in KB was spotted in quadruplicate and in a grid pattern onto potato dextrose agar (Sigma-Aldrich), followed by incubation at 37°C for 48 h. A 7-mm plug of R. solani AG4 was added central to the bacterial spots, followed by incubation at 25°C for 96 h. Inhibition of fungal growth was then assessed visually using a qualitative rating scale. Experiments were performed at least twice in triplicate.
In vitro growth curves.
Bacterial growth rates were determined in LB medium, defined medium (DM) (0.25× M63 salts supplemented with 0.2% glucose, 0.4% glycerol, 1 mM MgSO4, thiamine [1 μg/ml], and leucine, isoleucine, valine, tryptophan, glutamic acid, and glutamine [40 μg/ml each]) (70), NGM, and peptone glucose sorbitol (PGS; 1% Bacto peptone, 1% NaCl, 1% glucose, 0.15 M sorbitol) broths. Growth of 200-μl cultures normalized to an optical density at 600 nm (OD600) of 0.01 was analyzed in triplicate at 37°C in a microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA). The specific growth rate/h (μ) was calculated by using the formula: μ = 0.6931/doubling time (in hours).
Additional phenotypic characteristics.
Congo red binding properties of the strains were determined on LB agar plates containing Congo red dye (0.01% [wt/vol]) (6), incubated at 37°C for 24 to 78 h. The antibiotic activity was determined from spot cultures grown for 24 h at 37°C, killed by chloroform exposure, and then overlaid with Micrococcus luteus culture in LB top agar (0.7% agar). The zone of inhibition of growth of M. luteus was measured after 24 h at 37°C. Biofilm production for AU1054 was determined by optimization of previously described methods (18, 55). Strains grown for 18 h at 37°C on tryptic soy agar were suspended in LB medium containing 0.6% yeast extract and 0.8% glucose (LBYG) to an OD520 of ∼0.6 and serial 10-fold diluted in LBYG, and then 200 μl of the 10−3 and 10−4 dilutions were added to polystyrene microtiter plates (catalog no. 3595; Corning). After 24 h at 37°C, the growth was measured as OD540 by using a SpectraMax M5 microtiter plate reader (Molecular Devices). Biofilms were stained with crystal violet, 200 μl of 33% acetic acid was added to release the stain, and the biofilm was measured as the absorbance at 590 nm. Three biological and technical repeats were done for each sample. Biofilm production by each mutant was compared to wild-type AU1054 by using the Student t test. The production of siderophore activity was performed as described previously (63) except that a chrome azural S solution was added to the LB agar. Protease activity was assayed on skim milk agar (LB agar containing 1% skim milk) from spot cultures grown for 72 h at 37°C. Experiments were performed at least twice in triplicate.
RESULTS
Identification of genes required for AU1054 pathogenicity to nematodes.
Fifteen virulence-attenuated mutants were isolated by screening 3,000 mutants in 10 independent experiments. Southern analysis revealed that all but one mutant contained a single HimarGm insertion, and no gross chromosomal rearrangements were detected by BOX- and REP-PCR analyses (data not shown). The mutant containing the double insertion was not analyzed further. Nematode survival was significantly increased from 0% on AU1054 to 8.4 to 70.1% on the mutants after 72 h. Nematode survival was 42.8% on PA14 (positive) and 92.5% on OP50 (negative) (Table 3).
TABLE 3.
B. cenocepacia virulence-attenuated mutants
| Straina | Disrupted gene | Mean nematode survival ± SE at day 3b (%) | LT50 (days) | Predicted functionc |
|---|---|---|---|---|
| B. cenocepacia | ||||
| AU1054 (wt) | NA | 0 ± 0 | 1 | NAd |
| BPV134 | Bcen_1509 | 10.5 ± 4.2 | 2 | Unknown, predicted to be a secreted protein |
| BPV189 | trpB (Bcen_4409) | 26.8 ± 1.0 | 3 | Tryptophan synthase subunit beta |
| BPV419* | bnvR (Bcen_3021) | 30.7 ± 5.2 | 3 | Unknown, predicted to contain a helix-turn-helix DNA-binding motif |
| BPV522 | Bcen_2123 | 14.8 ± 4.6 | 2 | Unknown, FAD-oxidase-like |
| BPV1017* | nhaX (Bcen_0002) | 16.2 ± 1.6 | 2 | Sodium/hydrogen exchanger, COG0475-Kef-type K+ transport systems |
| BPV1367* | argG (Bcen_4651) | 17.7 ± 2.9 | 3 | Argininosuccinate synthase, COG0137 |
| BPV1406 | Bcen_3147 | 8.5 ± 5.4 | 2 | Unknown, contains AAA ATPase domain |
| BPV1573 | paaA (Bcen_2776) | 10.8 ± 5.5 | 2 | Phenylacetate-coenzyme A oxygenase |
| BPV1599 | leuS (Bcen_0171) | 14.7 ± 4.2 | 2 | Leucyl tRNA synthetase |
| BPV1852* | aroA (Bcen_0566) | 59.1 ± 13.5 | 4 | 5-Enolpyruvylshikimate-3-phosphate synthase COG0128 |
| BPV2031 | Bcen_1489 | 13.8 ± 4.7 | 2 | LysR-family transcriptional regulator |
| BPV2225* | gspJ (Bcen_0015) | 49.5 ± 11.3 | 3 | Type II secretion system, protein J, COG4795 (pulJ) |
| BPV2246* | purF (Bcen_4402) | 70.1 ± 5.6 | 5 | Amidophosphoribosyltransferase, COG0034 |
| BPV2391 | Bcen_2245 | 27.2 ± 13.7 | 2 | Unknown, contains UBA/THIF-type NAD/FAD binding fold |
| P. aeruginosa PA14 | NA | 42.8 ± 3.4 | 3 | Positive control for nematode assays |
| E. coli OP50 | NA | 92.5 ± 1.7 | NA | Negative control for nematode assays |
*, Mutant successfully complemented by expressing the respective wild-type gene in trans that caused a significant restoration of pathogenicity to nematodes.
That is, the mean of three independent experiments. Each experiment was performed with three replicates; approximately 50 nematodes were used for each replicate.
COG, cluster of orthologous groups.
NA, not applicable.
Determination of the DNA sequence flanking the HimarGm insertions revealed genes predicted to encode the GspJ T2SS pseudopilin, an uncharacterized sodium/proton exchanger, a LysR-family transcriptional regulator, a leuS tRNA synthetase, five hypothetical proteins of unknown function (locus tags Bcen_1509, Bcen_3021, Bcen_2123, Bcen_3147, and Bcen_2245) and five metabolic enzymes (trpB, argG, paaA, aroA, and purF) (Table 3). The paaA gene (Bcen_2776) encodes the phenylacetate-coenzyme A oxygenase subunit required for the ring opening reaction of the phenylacetic acid catabolic pathway previously identified as necessary for B. cenocepacia K56-2 pathogenicity to nematodes (43). This gene was also found to be induced under CF conditions relative to soil (73). The LysR regulator is a paralog to the shiny variant regulator, ShvR (an AU1054 ortholog of ShvR is encoded by Bcen_5634) previously identified as being required for K56-2 virulence to alfalfa and rough colony morphology (6). The protein encoded by Bcen_1509 is predicted to be secreted, and related proteins were only detected in 18 of the 60 draft and completed Burkholderia genome sequences present on the Integrated Microbial Genome Database (51). The six mutants that were successfully complemented with their respective wild-type genes expressed in trans (see below) are the focus of the remainder of the present study.
BPV419 was found to contain a HimarGm insertion into Bcen_3021, which is predicted to encode a hypothetical protein of unknown function and is 6 bp upstream of Bcen_3022, which is also predicted to encode another protein of unknown function. A helix-turn-helix motif was detected in amino acids 191 to 211 of the predicted protein encoded by Bcen_3021 using HTH and GYM 2.0 algorithms (22, 53), suggesting that this protein may bind DNA and regulate gene expression. We named Bcen_3021, bnvR, for Burkholderia nematode virulence regulator. An orthologous predicted protein 100% identical is present in the B. cenocepacia HI2424 genome, which is an environmental strain of the same multilocus sequence type (ST-122) as AU1054 (44). Another ortholog (94.2% identical) was detected in the genome of B. ambifaria MC40-6 but was not detected in the genomes of B. cenocepacia strains MC0-3, J2315, and PC184 and 55 other Burkholderia genomes currently present on the Integrated Microbial Genome Database (51).
In contrast, the other five virulence-attenuated mutants that were successfully complemented contained HimarGm mutations in genes that are conserved in all 60 Burkholderia genomes currently available in the Integrated Microbial Genome Database. BPV2225 was found to have an insertion in Bcen_0015 predicted to encode general secretion protein J (gspJ), a component of the type 2 secretion system (T2SS). The T2SS is evolutionarily related to type IV pili, and GspJ is a minor pseudopilin whose molecular function for general secretion is not well understood; however, GspJ is known to be essential for T2SS function (3, 16, 61). The T2SS is used by pathogenic and environmental bacteria to secrete a variety of toxins and enzymes. The T2SS is necessary for secretion of protease, lipase, and phospholipase C exoenzymes in B. pseudomallei but not for virulence in a hamster model of melioidosis (21). Virulence factors such as two predicted polygalacturonases (Bcen_4887 and Bcen_5664) and two zinc metalloproteases (ZmpA [Bcen_1233] and ZmpB [Bcen_3302]), which are known to be important for pathogenicity to onions and in the rat agar bead model of chronic infection, respectively (27, 40), are likely secreted by T2SS (1).
BPV1017 contained an insertion in Bcen_0002 predicted to encode a protein containing a putative sodium-proton exchanger domain (Kef-type K+ transport system, E-value 4e−04) (Table 3, Fig. 1). Sodium-proton exchangers are known to be involved in maintaining pH and ionic homeostasis (30, 56). This gene was named nhaX for Na/H antiporter.
FIG. 1.
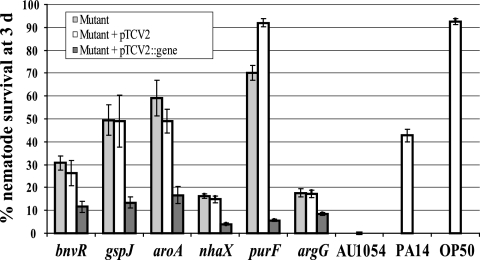
Attenuated virulence of AU1054 mutants to C. elegans on NGM. Six mutants were significantly (P < 0.05) attenuated in virulence to C. elegans at 3 days. Light gray bars represent mutant strains, white bars are mutant strains containing an empty pTCV2 vector, and dark gray bars are mutant strains containing pTCV2 plus complementing gene. The disrupted genes are denoted in parentheses below each mutant. The y axis shows the percent nematode survival on lawns of the strains 3 days after the addition of early L4 nematodes, and the x axis indicates the bacterial strains. B. cenocepacia AU1054 (wild type), P. aeruginosa PA14 (positive control), and E. coli OP50 (negative control) are also shown. Error bars indicate standard error of the mean.
The remaining three virulence-attenuated mutants identified in the present study contained mutations in conserved central metabolic genes. BPV1367 contained a HimarGm inserted into argG (Bcen_4651) predicted to encode a protein that functions as an argininosuccinate synthase (COG0137). For Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, and Listeria monocytogenes, mutations in genes required for arginine biosynthesis result in severe attenuation of virulence, suggesting the importance of arginine metabolism for in vivo growth and virulence (28, 39, 47).
BPV1852 contained an insertion in aroA (Bcen_0566), predicted to function as a 5-enolpyruvylshikimate-3-phosphate synthase, EPSPS (COG0128), required for the biosynthesis of the chorismate precursor (shikimate pathway) for aromatic amino acid synthesis. EPSPS is present in bacteria, plants, and fungi and is the target of the herbicide glyphosate (65). For several pathogens, mutations in the shikimate pathway result in severely attenuated virulence, and these mutants have been of great utility for the generation of live vaccines (7, 33, 58). The physiological basis for attenuated virulence of aroA strains is not completely known and can be due to the inability to synthesize p-aminobenzoic acid and folic acid (67), aromatic amino acids (54), or menaquinone required for aerobic respiration (68).
BPV2246, the most severely attenuated mutant in pathogenicity to nematodes, was found to contain an insertion in purF (Bcen_4402), predicted to encode a protein that functions as a amidophosphoribosyltransferase (COG0034), the first step in the de novo synthesis of purines. Like mutations in the shikimate pathway, mutations in the de novo synthesis of purines result in severe attenuation of virulence for some pathogenic bacteria (37, 52, 59).
Complementation of AU1054 virulence-attenuated mutants.
As mentioned above, six of the mutants were successfully complemented by expressing their respective wild-type genes in trans (Fig. 1A, Table 3). Nematode survival on lawns of the six complemented mutants was significantly less (P < 0.05) than for the mutant or the mutant containing the empty vector. Mutants with the greatest virulence defect—BPV2246 (purF), BPV2225 (gspJ), and BPV1852 (aroA)—which showed 70, 49.5, and 59% worm survival, respectively, at 3 days showed the greatest restoration of virulence by complementation with 5.6, 13.4, and 16.7% worm survival, respectively, at 3 days. Complementation of the other three mutants BPV419 (bnvR), BPV1017 (nhaX), and BPV1367 (argG), which showed 30.5, 16.3, and 17.6% worm survival, respectively, at 3 days showed significant (P < 0.05) restoration of the virulence and reduced survival of nematodes to 11.5, 4, and 8.4%, respectively, at 3 days.
Characterization of attenuated pathogenicity to C. elegans.
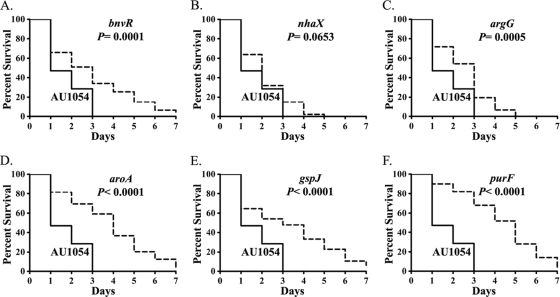
To better understand the virulence characteristics of the strains, we compared the Kaplan-Meier nematode survival plots for each mutant to the wild-type AU1054. All of the mutants, except BPV1017 (nhaX), showed significantly (P < 0.05) greater nematode survival over 7 days than did AU1054 (Fig. 2). As expected, the mutants displaying the greatest virulence defect at 3 days, e.g., BPV2246 (purF), BPV2225 (gspJ), and BPV1852 (aroA), also showed significantly higher median survival times (LT50) of 5, 3, and 4 days, respectively, than did wild-type AU1054 (1 day). Both the mutants BPV1367 (argG) and BPV419 (bnvR) showed LT50 of 3 days. However, BPV1017 (nhaX) (P = 0.0653) was not significantly different from AU1054 in the Kaplan-Meier survival analysis but showed a higher LT50 of 2 days. All mutants exhibited pathogenicity to nematodes resembling a slow kill reaction by PA14 (LT50 = 3 days), where 0 to 18% of animals were surviving at 7 days.
FIG. 2.
The virulence of the mutants is attenuated compared to the wild-type B. cenocepacia AU1054 in the C. elegans infection model. Kaplan-Meier survival plots of C. elegans nematodes (n = 450) on BPV419 (bnvR) (A), BPV1017 (nhaX) (B), BPV 1367 (argG) (C), BPV1852 (aroA) (D), BPV2225 (gspJ) (E), and BPV2246 (purF) (F) compared to the wild-type AU1054 are shown. AU1054 is indicated by solid lines, and mutants are indicated by dashed lines. All of the experiments were performed three independent times, each time in triplicate with approximately 50 nematodes on each plate. A log-rank test was used to compare survival differences and calculate the probability (P).
In summary, five of six mutants with significant attenuation of nematode pathogenicity after 3 days were also significantly different from the wild type up to 7 days, where they exhibited a slow kill reaction with nematodes compared to the fast kill of the wild type.
Kaplan-Meier survival analysis revealed significant (P < 0.05) reduction in worm LT50 for five of six complemented mutants compared to the mutants or mutants with empty vector pTCV2. Complementing the mutants with the respective gene reduced the LT50 from 5 to 2 days for BPV2246 (purF); from 4 to 2 days for BPV1852 (aroA); and from 3 to 2 days for BPV2225 (gspJ), BPV1367 (argG), and BPV419 (bnvR) (data not shown). Complemented BPV1017 (nhaX) showed a nonsignificant (P > 0.05) reduction in LT50 from 2 days to 1 day.
Pathology of wild-type and mutant AU1054-infected C. elegans.
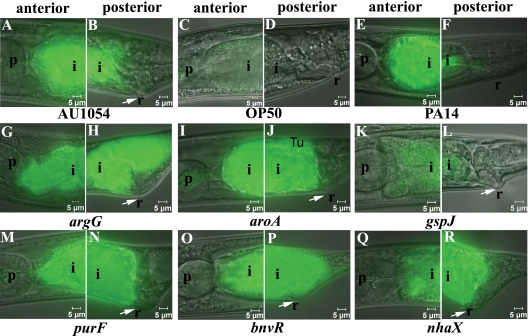
To understand how AU1054 exhibits rapid pathogenicity to C. elegans nematode on NGM, we observed nematodes for infection and other pathologies by using GFP-labeled wild-type and mutant AU1054 strains, E. coli OP50, and P. aeruginosa PA14 (Fig. 3). AU1054-infected nematodes showed reduced growth, severe intestinal infection, distended intestinal lumen, and swollen rectum at 24 to 48 h (Fig. 3A and B) in all nematodes observed (27/27), whereas no or few bacterial cells were observed in the intestines of nematodes associated with OP50 (Fig. 3C and D). P. aeruginosa PA14 caused a less prevalent infection of posterior nematode intestine and no rectal swelling (Fig. 3E and F). The swollen rectum, also referred to as the deformed anal region (Dar), phenotype caused by AU1054 infection is unusual because it is known to be caused by Microbacterium nematophilum that infects the rectum and Dar is thought to be an innate immune response to rectal tissue colonization (32). Fluorescent AU1054 cells were eliminated from nematodes transferred to OP50, suggesting that AU1054 establishes a transient infection (data not shown). Nematodes observed from lawns of mutant strains BPV419 (bnvR) (19/20), BPV1017 (nhaX) (22/22), BPV1367 (argG) (18/21), BPV1852 (aroA) (18/18), and BPV2246 (purF) (17/17) showed severe infection and pathology similar to the wild-type AU1054 (Fig. 3G to J and M to R). However, most of the observed nematodes (19/24) from the BPV2225 (gspJ) mutant lawns showed weakly infected intestines (Fig. 3K to L) compared to the wild-type AU1054 but still showed a swollen rectum. In summary, AU1054 and all mutants except BPV2225 (gspJ) caused severe pathology to C. elegans, including rectal swelling.
FIG. 3.
Pathology of C. elegans infected by AU1054 mutants on NGM lawns at 24 to 48 h. Images are overlays of Nomarski and epifluorescence micrographs of the anterior and posterior C. elegans intestine at 24 to 48 h after exposure to GFP-labeled strains. The epifluorescent micrographs were taken using the same exposure (200 ms); hence, the absence of green color indicates the lack of GFP-labeled bacterial strains, and the presence of green color indicates infection of the anterior and posterior intestine. The positive control AU1054 (A and B), negative control OP50 (C and D), and P. aeruginosa PA14 (E and F) are shown. E. coli OP50 did not infect the nematodes. Mutants BPV1367 (argG) (G and H), BPV1852 (aroA) (I and J), BPV2246 (purF) (M and N), BPV419 (bnvR) (O and P), and BPV1017 (nhaX) (Q and R) infected the nematodes similar to wild-type AU1054, whereas strain BPV2225 (gspJ) (K and L) showed reduced infection of nematode intestines. Infected C. elegans nematodes showed stunted growth, distended intestinal lumen, and deformed anal region (indicated by arrows) compared to E. coli. The pharynx (p), intestine (i), and rectum (r) are indicated.
Onion pathogenicity and antifungal activity.
Since AU1054 is a multihost pathogen that exhibits high pathogenicity to onion and produces strong antifungal activity, these characteristics were assessed for the virulence-attenuated mutants isolated in the nematode model (Table 4). Three of the six mutants—BPV1367 (argG), BPV1852 (aroA), and BPV2246 (purF)—showed significantly decreased antifungal activity compared to AU1054. Three of the six mutants—BPV1852 (aroA), BPV2225 (gspJ), and BPV2246 (purF)—showed significantly reduced pathogenicity to onion, measured as a smaller area of macerated tissue. The reduced pathogenicity to onion by BPV2225 (gspJ) is likely due to the inability to secrete endopolygalacturonase(s), which utilize the T2SS (43). The defect in secreted polygalacturonase activity was confirmed by a lack of polygalacturonic acid degradation by mutant BPV2225 (gspJ) on Hildebrand's medium (Table 4 and Fig. 4) and also for the two other mutants, BPV1852 (aroA) and BPV2246 (purF), attenuated in virulence to onion (Table 4). BPV1367 (argG) also showed a significant reduction in polygalacturonic acid degradation, although the area of onion maceration by this mutant was not different from AU1054. It is important to note that, as in the nematode assays for virulence, the inocula contained high numbers of cells so that defects in onion virulence or antifungal activity are not simply due to an absence of mutant cells on the assay media. In contrast, a mutation in bnvR and nhaX was normal in pathogenicity to onion and production of antifungal activity. In summary, four genes (argG, aroA, gspJ, and purF) were determined to be required for virulence in multiple hosts, whereas bnvR and nhaX functions only in virulence to nematode or animal hosts.
TABLE 4.
Phenotypic characterization of AU1054 mutants attenuated in pathogenicity to C. elegans
| Characteristic | Straina |
||||||
|---|---|---|---|---|---|---|---|
| AU1054 (wild type) | BPV419 (bnvR) | BPV1017 (nhaX) | BPV1367 (argG) | BPV1852 (aroA) | BPV2225 (gspJ) | BPV2246 (purF) | |
| Antifungal activityb | 3.6 ± 0.1 | 3.3 ± 0.2 | 3.3 ± 0.1 | 2.7 ± 0.1 | 2.4 ± 0.2 | 3.4 ± 0.1 | 3 ± 0.1 |
| Virulence against onion (area, mm2)c | 914.3 | 860.7 | 694.7 | 784.8 | 645.7* | 440.7** | 406.0** |
| Polygalacturonic acid degradationd | 26.3 | 27.0 | 26.6 | 20.0* | 14.7** | 11.1** | 21.0* |
| Growth rate/h (μ) in rich medium (LB) | 0.64 | 0.64 | 0.64 | 0.62 | 0.60 | 0.40 | 0.15 |
| Growth rate/h (μ) in minimal medium (DM) | 0.15 | 0.14 | 0.14 | NDe | ND | 0.08 | ND |
| Growth rate/h (μ) in assay medium (NGM) | 0.13 | 0.13 | 0.11 | 0.13 | ND | 0.09 | ND |
| Growth rate/h (μ) in assay medium (PGS) | 0.43 | 0.44 | 0.45 | 0.44 | 0.14 | 0.44 | 0.42 |
| Biofilm formationf (biofilm/growth) | 0.50 ± 0.09 (0.49 ± 0.09) | 0.57 ± 0.07 (0.59 ± 0.08) | 0.61 ± 0.05 (0.62 ± 0.06) | 0.42 ± 0.06 (0.43 ± 0.09) | 0.68 ± 0.06 (0.65 ± 0.06) | 0.60 ± .07 (0.57 ± 0.06) | 0.23 ± 0 .04 (0.31 ± 0.04) |
| Siderophore productiong | +++ (3 mm) | +++ (3 mm) | +++ (3 mm) | +++ (3 mm) | ++ (1.6 mm) | +++ (3 mm) | ND |
| Antibiotic activityh | ++ (2 mm) | ++ (2 mm) | ++ (2 mm) | ++ (2 mm) | + (1 mm) | ++ (2 mm) | ND |
Phenotypic characteristics significantly different from those of AU1054 are indicated in boldface type.
Based on a mean rating scale (0 to 4) ± the standard error of the mean.
Presented as the area (in mm2) of maceration determined with an absolute digimatic caliper; treatment means were separated by using a two-tailed t test. *, P < 0.05; **, P < 0.01.
Mean diameter of degradation zone of 12 replicates from three experiments spotted onto Hildebrand's medium. Treatment means were separated by using a two-tailed t test. *, P < 0.05; **, P < 0.01.
No growth detected.
Absorbance at 590 nm of crystal violet stain (A590 crystal violet stain/A540 planktonic culture). Means and standard errors of mean from 1 representative experiment are indicated in parentheses. Only BPV2246 developed biofilms that were significantly different from AU1054 in all three experiments.
Diameter (in mm) of clearing of chrome azural S stain.
Diameter (in mm) of growth inhibition of Micrococcus luteus.
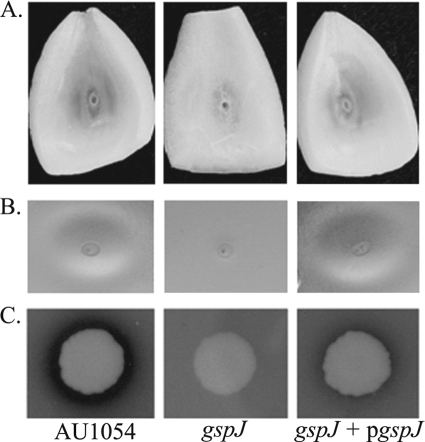
FIG. 4.
Role for gspJ in secretion of exoenzymes. The mutant BPV2225 (gspJ) shows reduced onion maceration (A), polygalacturonic acid degradation (B), and protease activity on skimmed milk agar (C) compared to AU1054 (wild type) and restoration of these activities by expressing gspJ in trans (gspJ + pgspJ).
In vitro growth characteristics of the virulence-attenuated mutants.
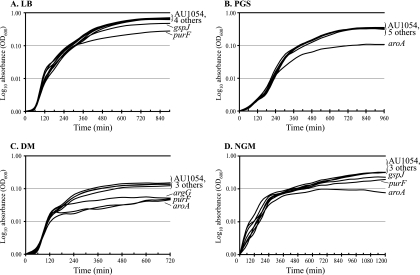
Growth characteristics of the mutant and wild-type AU1054 strains was assessed in rich medium (LB medium), defined minimal media (DM), and the nematode virulence assay media NGM and PGS (Fig. 5). In LB medium, strains BPV2225 (gspJ) and BPV2246 (purF) had significantly lower growth rates and reached a lower final density than AU1054 (Fig. 5A, Table 4). BPV1852 (aroA) had a slight decrease in growth rate and reached slightly lower density in LB than wild type, while the other three strains were not significantly different than AU1054. In PGS, only BPV1852 (aroA) showed reduced growth (Fig. 5B). In DM, strains BPV1367 (argG), BPV1852 (aroA), and BPV2246 (purF) failed to grow and are likely auxotrophs. BPV2225 (gspJ) grew more slowly and reached a lower density in DM than AU1054. BPV419 (bnvR) and BPV1017 (nhaX) did not differ significantly in the growth rate and maximum density from that reached by AU1054 (Fig. 5C). In NGM, BPV1852 (aroA) failed to grow, whereas BPV2225 (gspJ) and BPV2246 (purF) showed a reduced growth rate and reached a lower final density than the rest of the strains AU1054, BPV1367 (argG), BPV1017 (nhaX), and BPV419 (bnvR) (Fig. 5D).
FIG. 5.
Growth characteristics of the virulence attenuated AU1054 mutants. (A) In LB medium, gspJ and purF were defective. (B) In PGS, only aroA was defective. (C) In DM, argG, purF, and aroA were defective. (D) In NGM, aroA, purF, and gspJ were defective. The values represented are the mean of two independent experiments, each performed in triplicate until the growth curves reached a plateau.
In summary, BPV1852 (aroA) showed a severe growth defect on all tested media except LB medium. BPV2246 (purF) showed a severe to moderate growth defect on all media except PGS. BPV2225 (gspJ) showed a moderate growth defect on all of the tested media except PGS, whereas BPV1367 (argG) was defective only on DM. The other strains, BPV419 (bnvR) and BPV1017 (nhaX), did not exhibit any significant growth defect and were similar to the wild-type AU1054.
Additional phenotypic characteristics of the virulence-attenuated mutants.
Phenotypic variants of B. cenocepacia are known to occur with altered virulence characteristics and Congo red binding characteristics (15). Colonies of mutant strains absorbed Congo red to the same extent as AU1054, indicating that they are not variants of this phenotype (data not shown). BPV2246 (purF) was the only mutant strain found to reproducibly form a reduced biofilm (Table 4). Four of six mutants produced siderophore and antibiotic activities at levels similar to those of AU1054. BPV2246 (purF) produced no detectable siderophore or antibiotic activities. BPV1852 (aroA) produced lower siderophore and antibiotic activities than AU1054. The defect of these two mutants was not due to lack of growth since both BPV2246 and BPV1852 formed colonies on the assay media (data not shown).
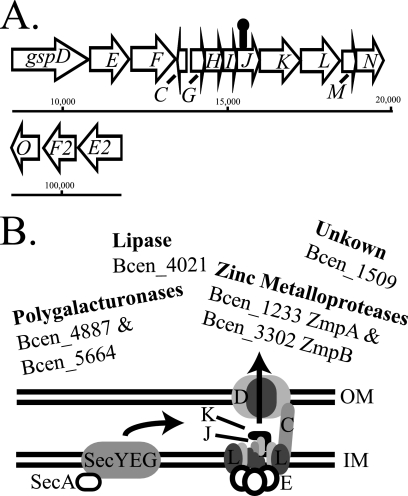
BPV2225 (gspJ) was found defective in onion maceration and degradation of polygalacturonic acid; therefore, we determined whether there is also a defect in protease activity and whether these activities are restored by expressing gspJ in trans. We found that BPV2225 (gspJ) is defective in casein hydrolysis in addition to onion maceration and polygalacturonic acid degradation and that these activities are restored by the expression of gspJ (Fig. 4). These results suggest that effectors secreted by the T2SS are required for virulence in both nematodes and onion but not for the production of antifungal or antibiotic activities. In addition, this suggests that the four T2SS genes, gspKLMN, downstream of gspJ are expressed in the BPV225 (gspJ) mutant, possibly from a secondary promoter (Fig. 6). Additional experiments are in progress to determine the optimal conditions for T2SS expression and proteomic analysis of the T2SS secretome (our preliminary results suggest that protease activity is enhanced in DM and NGM relative to LB).
FIG. 6.
Physical map and functional model of the AU1054 T2SS. (A) The majority of the genes encoding the T2SS are present in a single region of chromosome one. The HimarGm transposon (lollipop) located gspJ is not likely to be polar onto gspKLMN since these genes are also known to be essential for T2SS and expression of gspJ alone restores virulence and exoenzyme secretion to the BPV2225 (gspJ) mutant. The prepilin peptidase gene (gspO) and paralogs gspE2 and gspF2 are located in a second region of chromosome one. GspO is known to be essential for T2SS, however, the functions of the paralogs is unknown. (B) Model of T2SS in AU1054. T2SS proteins require the Sec pathway for secretion into the periplasm, where they are folded. By a poorly understood mechanism, proteins are recognized by GspK for secretion through the outer membrane pore GspD. GspJ interacts with two other pseudopilins, GspI and GspG, all of which are required for T2SS. Proteins possibly secreted by AU1054 T2SS are two predicted polygalacturonases (Bcen_4887 and Bcen5664), zinc metalloproteases (Bcen_1233 and Bcen_3302), nonhemolytic phospholipase C (Bcen_4021), and unknown proteins such as Bcen_1509 identified in the present study as having a role in AU1054 pathogenicity to nematodes. OM, outer membrane; IM, inner membrane.
In summary, BPV419 (bnvR) and BPV1017 (nhaX) displayed phenotypes identical to that of AU1054 except for pathogenicity to nematodes. BPV1852 (aroA) and BPV2246 (purF) were defective in pathogenicity to onion and growth in minimal medium and were reduced in antifungal, siderophore, and antibiotic activities, and BPV2246 also showed reduced ability to form biofilms. BPV2225 (gspJ) was reduced in pathogenicity to onion and growth in most media except PGS but similar to AU1054 in the production of antifungal, siderophore, and antibiotic activities and biofilm formation. BPV1367 (argG) was defective in pathogenicity to onion; the production of antifungal, siderophore, and antibiotic activities; and in growth in minimal medium. Thus, genes were identified that were required for the pathogenicity of AU1054 to multiple and specific hosts.
DISCUSSION
The objective of this study was to determine mechanisms that contribute to virulence and host specificity of the opportunistic pathogen, B. cenocepacia AU1054, a strain isolated from the blood of a person with CF that exhibits multihost pathogenicity (13, 64). Our study revealed that the T2SS pseudopilin gene, gspJ, and the biosynthetic genes argG, aroA, and purF are required for multihost pathogenicity, while the role for bnvR and nhaX in virulence is host specific. AU1054 is an ideal model organism because it is a clinical strain that shares a multilocus sequence typing genotype with known environmental strains isolated from onion fields in Michigan and New York (38, 44). Multihost pathogenicity of AU1054 similar to that of the environmental strains suggests limited evolution of pathogenicity of this strain during growth in the cystic fibrosis lung (64). In contrast, strains of the ET12 and Midwest epidemic clonal lineages exhibit variable degrees of pathogenicity to nematodes and contain independent mutations in virulence-related loci, including the T2SS (10, 34, 64; see also below). Clinical isolates lacking T2SS are common, suggesting that these are cheater strains that evolve during chronic lung infection and benefit from coinfection with T2SS-positive strains, while working less to secrete virulence factors.
Since AU1054 exhibits rapid pathogenicity to C. elegans under conditions where P. aeruginosa kills slowly (46), we utilized the nematode model to identify virulence mechanisms of AU1054. However, the mechanisms of fast killing by AU1054 are not fully understood. Quorum sensing and the quorum-sensing regulated protein AidA are known to be required for B. cenocepacia H111, an ET12 epidemic clonal strain, to infect and kill C. elegans on slow kill media (35, 41). Mutants defective in quorum sensing or Aid were not obtained in the present study, although we also expect them to be important for AU1054 virulence. The ring-opening enzymatic reactions of the phenylacetic acid catabolic pathway was also known to be required for B. cenocepacia K56-2 pathogenicity to nematodes (43), and our results confirm this finding in AU1054. However, we discovered additional genes required for virulence, which may reflect the adaptability of Burkholderia in host-bacterium interactions (48).
Evidence for the role of the T2SS for AU1054 pathogenicity was unexpected, because no role was found previously for the T2SS in B. pseudomallii, B. vietnamiensis, and B. cenocepacia H111 (21, 24, 41). T2SS was found to be required for B. gladioli pathogenicity to mushrooms (14). In addition, the genome sequence of the ET12 epidemic clone strain, J2315, revealed a 110-bp insertion in gspL required for T2SS function (34). Another ET12 strain, K56-2, has a functional T2SS required for the secretion of zinc metalloproteases, although other ET12 strains have different mutations in gspL, suggesting that loss of T2SS function is a trait derived in the CF lung (1, 34, 40). Clinical strains with defective T2SS may function as cheaters that benefit from secretion of virulence factors and exoenzymes of other bacteria inhabiting the CF lung. However, the T2SS was found to be induced by AU1054 in synthetic CF sputum relative to soil extract, indicating a possible role for virulence (74). The virulence defect of the gspJ mutant to onion can be explained by the inability to secrete two polygalacturonases, Bcen_4887 and Bcen_5664 (Fig. 6). It is not likely that the known nematode virulence factor, AidA, is T2SS dependent because it lacks a secretion signal, as determined by SignalP 3.0, and the T2SS is not required for H111 pathogenicity to nematodes (41). However, for the ET12 strain K56-2, the T2SS-dependent protease ZmpA is required for persistence in the rat lung, and both ZmpA and ZmpB are required for K56-2-induced inflammation in the same rat model (40). Furthermore, strain K56-2 with a functional T2SS was more virulent to nematodes and produces more protease activity than strain J2315 with a nonfunctional T2SS (10, 25). These observations suggest that the ancestral environmental B. cenocepacia had a functional T2SS during infection of the cystic fibrosis lung and that cheater strains with nonfunctional T2SS frequently occur during chronic lung infections. In summary, these results suggest that B. cenocepacia T2SS is important for virulence and might function by secreting known or novel virulence factors such as the protein encoded by Bcen_1509 identified in the present study (Fig. 6). We are currently conducting experiments to determine the T2SS effectors required for AU1054 pathogenicity.
In addition to gspJ, the genetic analysis of rapid killing of C. elegans caused by AU1054 revealed roles for two genes, bnvR and nhaX, in virulence that were previously unknown. bnvR is interesting because the predicted protein, identified as a hypothetical with unknown function, contains a helix-turn-helix motif, suggesting that it might bind DNA and regulate gene expression. Furthermore, only a few Burkholderia strains contain bnvR homologs, suggesting that this gene was acquired by horizontal transfer. The presence of a transcription factor is known to significantly affect host-bacterium interactions of a variety of bacteria (57). For example, a single regulatory protein is sufficient to alter the host range of vibrio symbionts (50), and a B. cenocepacia LysR regulatory protein affects virulence and phenotypic variation (6).
The discovery that nhaX is required for pathogenicity to nematodes is interesting because it is only distantly related to other studied sodium (cation)/hydrogen (proton) exchangers. NhaA (COG3064) and NhaB (COG0475) domain-containing proteins were not detected in AU1054. However, five predicted proteins contained NhaP (COG0025) and two contained NhaD (COG1055) domains. Sodium/hydrogen exchangers are known to regulate pH and ionic homeostasis and maintenance of the cell shape and to control phagosomal acidification (56, 66). The resting pH of the C. elegans intestine is pH 4, although this oscillates with the defecation cycle, where transcellular transport of proton ions by intestinal sodium/proton exchangers triggers muscle contraction (5). Thus, the nhaX predicted sodium/hydrogen exchanger may function by maintaining pH or osmotic homeostasis in the nematode intestine.
The AU1054 metabolic argF, aroA, and purF mutants showed growth defects on various media, and it is tempting to conclude that the attenuated virulence of these mutants could be attributed to lack of growth. However, evidence indicates that the attenuation in virulence is not due to lack of growth because (i) nematodes were exposed to visibly confluent bacterial lawns and therefore sufficient infective units; (ii) no visible difference in fluorescent bacteria infecting the nematode intestine was evident except for gspJ; (iii) aroA and purF mutants were defective in producing siderophore, antibiotics, and antifungal activities even though the mutants formed colonies on the assay media; and (iv) argG is defective in antifungal activity while still producing siderophore and antibiotic activities, suggesting that this gene is involved in the synthesis of an antifungal metabolite. All three auxotrophs show reduced pathogenicity to onion, suggesting that these metabolic functions are required in multiple hosts. Because aroA is only found in bacteria, plants, and fungi and is the target for the herbicide glyphosate, glyphosate or related compounds may be effective in inhibiting the growth of AU1054 in human, animal, or plant hosts.
In summary, C. elegans was used successfully to identify genes involved in multihost pathogenicity of the human opportunistic pathogen B. cenocepacia AU1054. A putative novel transcription factor, sodium/hydrogen exchanger, gspJ (T2SS), and three metabolic genes were determined to be required for rapid pathogenicity to C. elegans, and five of these were also required for multihost pathogenicity. However, some virulence factors, such as those encoded by bnvR identified in the present study and those encoding a nematode toxin, aidA, the outer membrane protein opcI, and a component of T3SS, hldA, appear to be host specific (71). We further illustrate here how much remains to be learned about the complex problem of how select environmental microbes function as opportunistic pathogens.
Acknowledgments
We gratefully acknowledge Michael Bagdasarian and A. Cody Springman for comments on the manuscript, John LiPuma and Jim Tiedje for providing strains and advice, Terry Marsh for assaying for genome rearrangement, Dieter Schifferli and Steven Lindow for providing strains, and R. Lucas Gray for help with experiments.
This study was funded by the Center for Microbial Pathogenesis at Michigan State University.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Abe, M., and T. Nakazawa. 1996. The dsbB gene product is required for protease production by Burkholderia cepacia. Infect. Immun. 64:4378-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bally, M., A. Filloux, M. Akrim, G. Ball, A. Lazdunski, and J. Tommassen. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6:1121-1131. [DOI] [PubMed] [Google Scholar]
- 4.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. [DOI] [PubMed] [Google Scholar]
- 5.Beg, A. A., G. G. Ernstrom, P. Nix, M. W. Davis, and E. M. Jorgensen. 2008. Protons act as a transmitter for muscle contraction in Caenorhabditis elegans. Cell 132:149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernier, S. P., D. T. Nguyen, and P. A. Sokol. 2008. A LysR-type transcriptional regulator in Burkholderia cenocepacia influences colony morphology and virulence. Infect. Immun. 76:38-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowe, F., P. O'Gaora, D. Maskell, M. Cafferkey, and G. Dougan. 1989. Virulence, persistence, and immunogenicity of Yersinia enterocolitica O:8 aroA mutants. Infect. Immun. 57:3234-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkholder, W. H. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115-118. [Google Scholar]
- 10.Cardona, S. T., J. Wopperer, L. Eberl, and M. A. Valvano. 2005. Diverse pathogenicity of Burkholderia cepacia complex strains in the Caenorhabditis elegans host model. FEMS Microbiol. Lett. 250:97-104. [DOI] [PubMed] [Google Scholar]
- 11.Chain, P. S., V. J. Denef, K. T. Konstantinidis, L. M. Vergez, L. Agullo, V. L. Reyes, L. Hauser, M. Cordova, L. Gomez, M. Gonzalez, M. Land, V. Lao, F. Larimer, J. J. LiPuma, E. Mahenthiralingam, S. A. Malfatti, C. J. Marx, J. J. Parnell, A. Ramette, P. Richardson, M. Seeger, D. Smith, T. Spilker, W. J. Sul, T. V. Tsoi, L. E. Ulrich, I. B. Zhulin, and J. M. Tiedje. 2006. Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73-Mbp genome shaped for versatility. Proc. Natl. Acad. Sci. U. S. A. 103:15280-15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, H., and D. M. Schifferli. 2007. Comparison of a fimbrial versus an autotransporter display system for viral epitopes on an attenuated Salmonella vaccine vector. Vaccine 25:1626-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, J. S., K. A. Witzmann, T. Spilker, R. J. Fink, and J. J. LiPuma. 2001. Endemicity and inter-city spread of Burkholderia cepacia genomovar III in cystic fibrosis. J. Pediatr. 139:643-649. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury, P. R., and J. A. Heinemann. 2006. The general secretory pathway of Burkholderia gladioli pv. agaricicola BG164R is necessary for cavity disease in white button mushrooms. Appl. Environ. Microbiol. 72:3558-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung, J. W., E. Altman, T. J. Beveridge, and D. P. Speert. 2003. Colonial morphology of Burkholderia cepacia complex genomovar III: implications in exopolysaccharide production, pilus expression, and persistence in the mouse. Infect. Immun. 71:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13:581-588. [DOI] [PubMed] [Google Scholar]
- 17.Ciche, T., and S. K. Goffredi. 2007. General methods to investigate microbial symbioses, p. 394-419. In C. R. Reddy, T. J. Beveridge, J. A. Breznak, G. A. Marzluf, T. Schmidt, and L. R. Snyder (ed.), Methods in general and molecular microbiology. ASM Press, Washington, DC.
- 18.Conway, B. A., V. Venu, and D. P. Speert. 2002. Biofilm formation and acyl homoserine lactone production in the Burkholderia cepacia complex. J. Bacteriol. 184:5678-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darby, C. 2005. Interactions with microbial pathogens. In The Caenorhabditis elegans research community. WormBook. doi: 10.1895/wormbook.1.21.1. [Online.] http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 20.Darby, C., C. L. Cosma, J. H. Thomas, and C. Manoil. 1999. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 96:15202-15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeShazer, D., P. J. Brett, M. N. Burtnick, and D. E. Woods. 1999. Molecular characterization of genetic loci required for secretion of exoproducts in Burkholderia pseudomallei. J. Bacteriol. 181:4661-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas, C. M., C. Guidi-Rontani, and R. J. Collier. 1987. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J. Bacteriol. 169:4962-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehlner-Gardiner, C. C., T. M. Hopkins, and M. A. Valvano. 2002. Identification of a general secretory pathway in a human isolate of Burkholderia vietnamiensis (formerly B. cepacia complex genomovar V) that is required for the secretion of hemolysin and phospholipase C activities. Microb. Pathog. 32:249-254. [DOI] [PubMed] [Google Scholar]
- 25.Gingues, S., C. Kooi, M. B. Visser, B. Subsin, and P. A. Sokol. 2005. Distribution and expression of the ZmpA metalloprotease in the Burkholderia cepacia complex. J. Bacteriol. 187:8247-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldmann, D. A., and J. D. Klinger. 1986. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J. Pediatr. 108:806-812. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez, C. F., E. A. Pettit, V. A. Valadez, and E. M. Provin. 1997. Mobilization, cloning, and sequence determination of a plasmid-encoded polygalacturonase from a phytopathogenic Burkholderia (Pseudomonas) cepacia. Mol. Plant-Microbe Interact. 10:840-851. [DOI] [PubMed] [Google Scholar]
- 28.Gordhan, B. G., D. A. Smith, H. Alderton, R. A. McAdam, G. J. Bancroft, and V. Mizrahi. 2002. Construction and phenotypic characterization of an auxotrophic mutant of Mycobacterium tuberculosis defective in l-arginine biosynthesis. Infect. Immun. 70:3080-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govan, J. R., P. H. Brown, J. Maddison, C. J. Doherty, J. W. Nelson, M. Dodd, A. P. Greening, and A. K. Webb. 1993. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet 342:15-19. [DOI] [PubMed] [Google Scholar]
- 30.Herz, K., S. Vimont, E. Padan, and P. Berche. 2003. Roles of NhaA, NhaB, and NhaD Na+/H+ antiporters in survival of Vibrio cholerae in a saline environment. J. Bacteriol. 185:1236-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrand, D. C. 1971. Pectate and pectin gels for differentiation of Pseudomonas sp. and other bacterial plant pathogens. Phytopathology 61:1430-1436. [Google Scholar]
- 32.Hodgkin, J., P. E. Kuwabara, and B. Corneliussen. 2000. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr. Biol. 10:1615-1618. [DOI] [PubMed] [Google Scholar]
- 33.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 34.Holden, M. T., H. M. Seth-Smith, L. C. Crossman, M. Sebaihia, S. D. Bentley, A. M. Cerdeno-Tarraga, N. R. Thomson, N. Bason, M. A. Quail, S. Sharp, I. Cherevach, C. Churcher, I. Goodhead, H. Hauser, N. Holroyd, K. Mungall, P. Scott, D. Walker, B. White, H. Rose, P. Iversen, D. Mil-Homens, E. P. Rocha, A. M. Fialho, A. Baldwin, C. Dowson, B. G. Barrell, J. R. Govan, P. Vandamme, C. A. Hart, E. Mahenthiralingam, and J. Parkhill. 2009. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 191:261-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber, B., F. Feldmann, M. Kothe, P. Vandamme, J. Wopperer, K. Riedel, and L. Eberl. 2004. Identification of a novel virulence factor in Burkholderia cenocepacia H111 required for efficient slow killing of Caenorhabditis elegans. Infect. Immun. 72:7220-7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 37.Ivanovics, G., E. Marjai, and A. Dobozy. 1968. The growth of purine mutants of Bacillus anthracis in the body of the mouse. J. Gen. Microbiol. 53:147-162. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs, J. L., A. C. Fasi, A. Ramette, J. J. Smith, R. Hammerschmidt, and G. W. Sundin. 2008. Identification and onion pathogenicity of Burkholderia cepacia complex isolates from the onion rhizosphere and onion field soil. Appl. Environ. Microbiol. 74:3121-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klarsfeld, A. D., P. L. Goossens, and P. Cossart. 1994. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE, and an arginine ABC transporter gene, arpJ. Mol. Microbiol. 13:585-597. [DOI] [PubMed] [Google Scholar]
- 40.Kooi, C., B. Subsin, R. Chen, B. Pohorelic, and P. A. Sokol. 2006. Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence. Infect. Immun. 74:4083-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kothe, M., M. Antl, B. Huber, K. Stoecker, D. Ebrecht, I. Steinmetz, and L. Eberl. 2003. Killing of Caenorhabditis elegans by Burkholderia cepacia is controlled by the cep quorum-sensing system. Cell. Microbiol. 5:343-351. [DOI] [PubMed] [Google Scholar]
- 42.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800-802. [PubMed] [Google Scholar]
- 43.Law, R. J., J. N. Hamlin, A. Sivro, S. J. McCorrister, G. A. Cardama, and S. T. Cardona. 2008. A functional phenylacetic acid catabolic pathway is required for full pathogenicity of Burkholderia cenocepacia in the Caenorhabditis elegans host model. J. Bacteriol. 190:7209-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LiPuma, J. J., T. Spilker, T. Coenye, and C. F. Gonzalez. 2002. An epidemic Burkholderia cepacia complex strain identified in soil. Lancet 359:2002-2003. [DOI] [PubMed] [Google Scholar]
- 45.Mahajan-Miklos, S., L. G. Rahme, and F. M. Ausubel. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 37:981-988. [DOI] [PubMed] [Google Scholar]
- 46.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 47.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 48.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 49.Mahenthiralingam, E., P. Vandamme, M. E. Campbell, D. A. Henry, A. M. Gravelle, L. T. Wong, A. G. Davidson, P. G. Wilcox, B. Nakielna, and D. P. Speert. 2001. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect. Dis. 33:1469-1475. [DOI] [PubMed] [Google Scholar]
- 50.Mandel, M. J., M. S. Wollenberg, E. V. Stabb, K. L. Visick, and E. G. Ruby. 2009. A single regulatory gene is sufficient to alter bacterial host range. Nature 458:215-21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markowitz, V. M., E. Szeto, K. Palaniappan, Y. Grechkin, K. Chu, I. M. Chen, I. Dubchak, I. Anderson, A. Lykidis, K. Mavromatis, N. N. Ivanova, and N. C. Kyrpides. 2008. The integrated microbial genomes (IMG) system in 2007: data content and analysis tool extensions. Nucleic Acids Res. 36:D528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFarland, W. C., and B. A. Stocker. 1987. Effect of different purine auxotrophic mutations on mouse-virulence of a Vi-positive strain of Salmonella dublin and of two strains of Salmonella typhimurium. Microb. Pathol. 3:129-141. [DOI] [PubMed] [Google Scholar]
- 53.Narasimhan, G., C. Bu, Y. Gao, X. Wang, N. Xu, and K. Mathee. 2002. Mining protein sequences for motifs. J. Comput. Biol. 9:707-720. [DOI] [PubMed] [Google Scholar]
- 54.O'Callaghan, D., D. Maskell, F. Y. Liew, C. S. Easmon, and G. Dougan. 1988. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect. Immun. 56:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 56.Padan, E., E. Bibi, M. Ito, and T. A. Krulwich. 2005. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717:67-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perego, M., and J. A. Hoch. 2008. Commingling regulatory systems following acquisition of virulence plasmids by Bacillus anthracis. Trends Microbiol. 16:215-221. [DOI] [PubMed] [Google Scholar]
- 58.Priebe, G. P., G. J. Meluleni, F. T. Coleman, J. B. Goldberg, and G. B. Pier. 2003. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect. Immun. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quarry, J. E., K. E. Isherwood, S. L. Michell, H. Diaper, R. W. Titball, and P. C. Oyston. 2007. A Francisella tularensis subspecies novicida purF mutant, but not a purA mutant, induces protective immunity to tularemia in mice. Vaccine 25:2011-2018. [DOI] [PubMed] [Google Scholar]
- 60.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 61.Sandkvist, M. 2001. Type II secretion and pathogenesis. Infect. Immun. 69:3523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, V. J. DiRita, and M. Bagdasarian. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 64.Springman, A. C., J. L. Jacobs, V. S. Somvanshi, G. W. Sundin, M. H. Mulks, T. S. Whittam, P. Viswanathan, R. L. Gray, J. J. Lipuma, and T. A. Ciche. 2009. Genetic diversity and multihost pathogenicity of clinical and environmental strains of Burkholderia cenocepacia. Appl. Environ. Microbiol. 75:5250-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinrucken, H. C., and N. Amrhein. 1980. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem. Biophys. Res. Commun. 94:1207-1212. [DOI] [PubMed] [Google Scholar]
- 66.Stewart, G. R., J. Patel, B. D. Robertson, A. Rae, and D. B. Young. 2005. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathol. 1:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stocker, B. A. 1990. Aromatic-dependent Salmonella as live vaccine presenters of foreign epitopes as inserts in flagellin. Res. Microbiol. 141:787-796. [DOI] [PubMed] [Google Scholar]
- 68.Stritzker, J., J. Janda, C. Schoen, M. Taupp, S. Pilgrim, I. Gentschev, P. Schreier, G. Geginat, and W. Goebel. 2004. Growth, virulence, and immunogenicity of Listeria monocytogenes aro mutants. Infect. Immun. 72:5622-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thongdee, M., L. A. Gallagher, M. Schell, T. Dharakul, S. Songsivilai, and C. Manoil. 2008. Targeted mutagenesis of Burkholderia thailandensis and Burkholderia pseudomallei through natural transformation of PCR fragments. Appl. Environ. Microbiol. 74:2985-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uehlinger, S., S. Schwager, S. P. Bernier, K. Riedel, D. T. Nguyen, P. A. Sokol, and L. Eberl. 2009. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect. Immun. 77:4102-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanlaere, E., A. Baldwin, D. Gevers, D. Henry, E. De Brandt, J. J. LiPuma, E. Mahenthiralingam, D. P. Speert, C. Dowson, and P. Vandamme. 2009. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int. J. Syst. Evol. Microbiol. 59:102-111. [DOI] [PubMed] [Google Scholar]
- 73.Yoder-Himes, D. R., P. S. Chain, Y. Zhu, O. Wurtzel, E. M. Rubin, J. M. Tiedje, and R. Sorek. 2009. Mapping the Burkholderia cenocepacia niche response via high-throughput sequencing. Proc. Natl. Acad. Sci. U. S. A. 106:3976-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoder-Himes, D. R., K. T. Konstantinidis, and J. M. Tiedje. Identification of potential therapeutic targets for Burkholderia cenocepacia by comparative transcriptomics. PloS One 5:e8724. [DOI] [PMC free article] [PubMed]
- 75.Zahariadis, G., M. H. Levy, and J. L. Burns. 2003. Cepacia-like syndrome caused by Burkholderia multivorans. Can. J. Infect. Dis. 14:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]