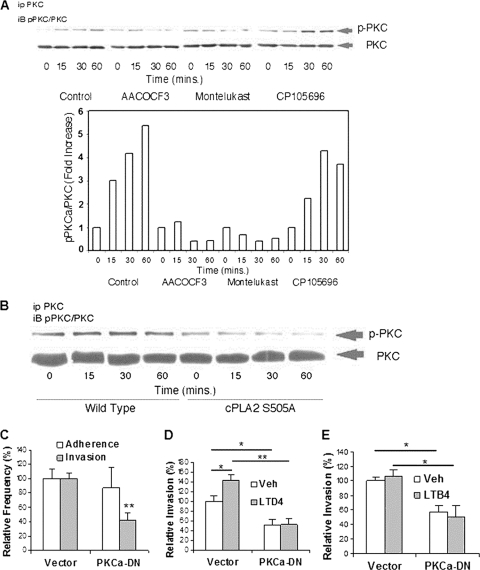
FIG. 8.
cPLA2α, 5-LO, and cysteinyl LTs contribute to E. coli invasion of HBMEC via PKCα. (A) HBMEC incubated with E. coli K1 strain RS218 at 37°C for various times in the presence of inhibitors/antagonists or the vehicle control were immunoprecipitated with PKCα antibody and then assessed for phospho-PKC by Western blotting with phospho-PKC antibody. The HBMEC lysates were also examined for the total amounts of PKC. The bands of phosphorylated and total PKC shown by a Western blot were calculated by ImageJ software, and ratios of phospho-PKC to PKC are expressed as fold increases compared to that at time zero. (B) Effect of cPLA2α S505A on PKCα activation. HBMEC transfected with the cPLA2α wild type or cPLA2α S505A were incubated with E. coli K1 strain RS218 for various times. The HBMEC lysates were immunoprecipitated with PKCα antibody and subsequently Western blotted with phospho-PKC antibody. The HBMEC lysates were examined for the total amounts of PKC as described above. (C) PKCα is involved in E. coli RS218 invasion of HBMEC. HBMEC transfected with the dominant negative PKCα construct or the vector control were used for bacterial binding and invasion assays. The data shown are means ± SEM from three separate experiments. Each experiment was performed in triplicate. **, P < 0.01 (Student's t test, comparison between the Ad5CA dominant negative PKCα [PKCa-DN] and the vector control). (D and E) Effects of LTD4 or LTB4 on E. coli K1 strain RS218 invasion of HBMEC transfected with the dominant negative PKCα construct or the vector control. HBMEC were pretreated with 1 μM LTD4 (D) or 1 μM LTB4 (E) for 30 min. Ethanol (0.5%) was used as the vehicle control (Veh). The data shown are means ± SEM from two independent experiments. Each experiment was performed in triplicate. *, P < 0.05; **, P < 0.01 (Student's t test, comparison between the Ad5CA dominant negative PKCα [PKCα-DN] and the vector control).