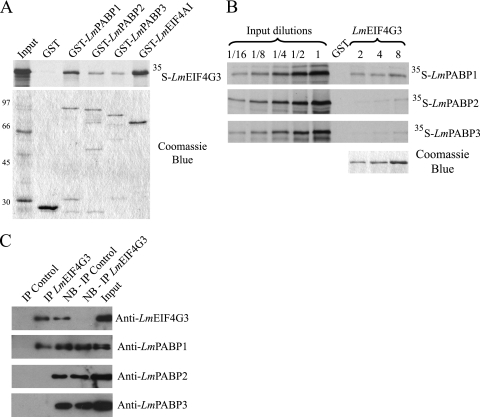
Fig. 6.
Analysis of the interaction between an L. major eIF4G homologue (LmEIF4G3) and LmPABP1, -2, and -3. (A) Pulldown assays between GST-PABP fusions and 35S-labeled LmEIF4G3. GST-LmEIF4AI was used as a positive control. On top is shown the autoradiography with the pulldown results, while below is shown the equivalent Coomassie-blue stained gel (molecular mass markers [kDa] are shown on the left). (B) Reverse pulldown assay with GST-LmEIF4G3 immobilized on the glutathione-Sepharose beads and incubated with the labeled LmPABP1, -2, and -3. Three different concentrations of GST-LmEIF4G3 (2, 4, and 8 μg, shown on the gel stained with Coomassie blue at the bottom right) were used in equivalent pulldown reactions with the three 35S-labeled proteins. For this assay, aliquots of the “Input” (equivalent to the same amount of labeled protein used in the pulldown reactions) plus serial dilutions (up to 16-fold) were also run on the gel and autoradiography for comparison with the amount of protein bound to the beads. In both panels A and B, GST alone bound to the beads was used as a negative control. (C) Immunoprecipitation of native LmEIF4G3, performed as described for Fig. 5. The product of the reaction, the nonbound (NB) supernatant, and the “Input” were then individually blotted with the anti-LmEIF4G3 serum and each of the three PABP antisera.