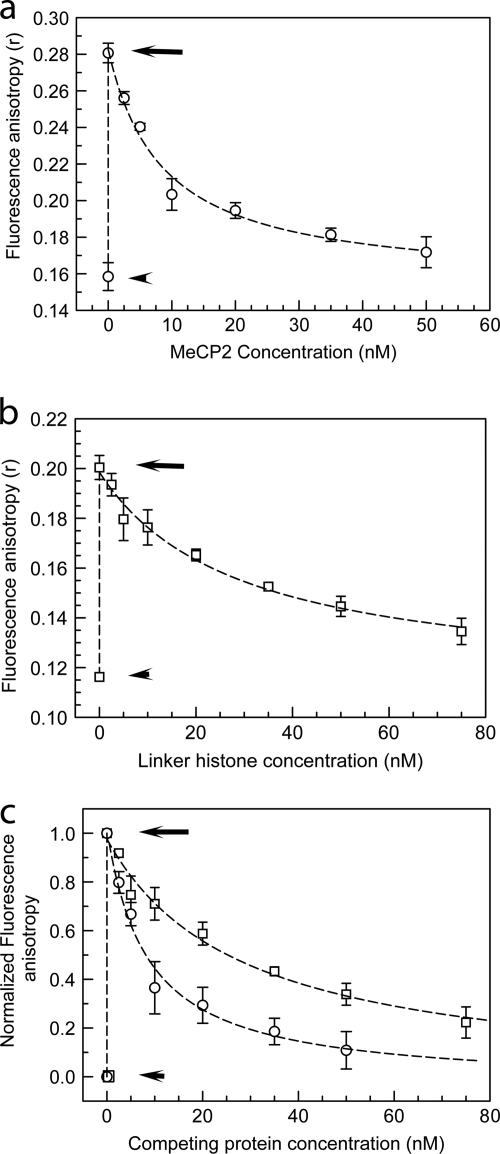
FIG. 5.
MeCP2 and histone H1 compete for nucleosome-binding sites in vitro. (a) Fluorescence anisotropy of 10 nM TMR-labeled H10 (circles) by itself (lower short arrow), upon incubation with an equimolar amount of 172-bp (the approximate chromatosome length) nucleosomes (upper long arrow), and upon incubation of the binary complex with increasing amounts of unlabeled MeCP2. (b) Same as for panel a, but with TMR-MeCP2 (squares) incubated with increasing amounts of unlabeled H10. (c) Normalized version of data shown in panels a and b. At an equimolar input of unlabeled MeCP2, ∼70% labeled H10 is excluded from mononucleosomes, whereas with unlabeled H10, ∼30% of labeled MeCP2 is competed out. Error bars represent SEM. Data are based on 3 separate acquisitions for each experiment type.