Abstract
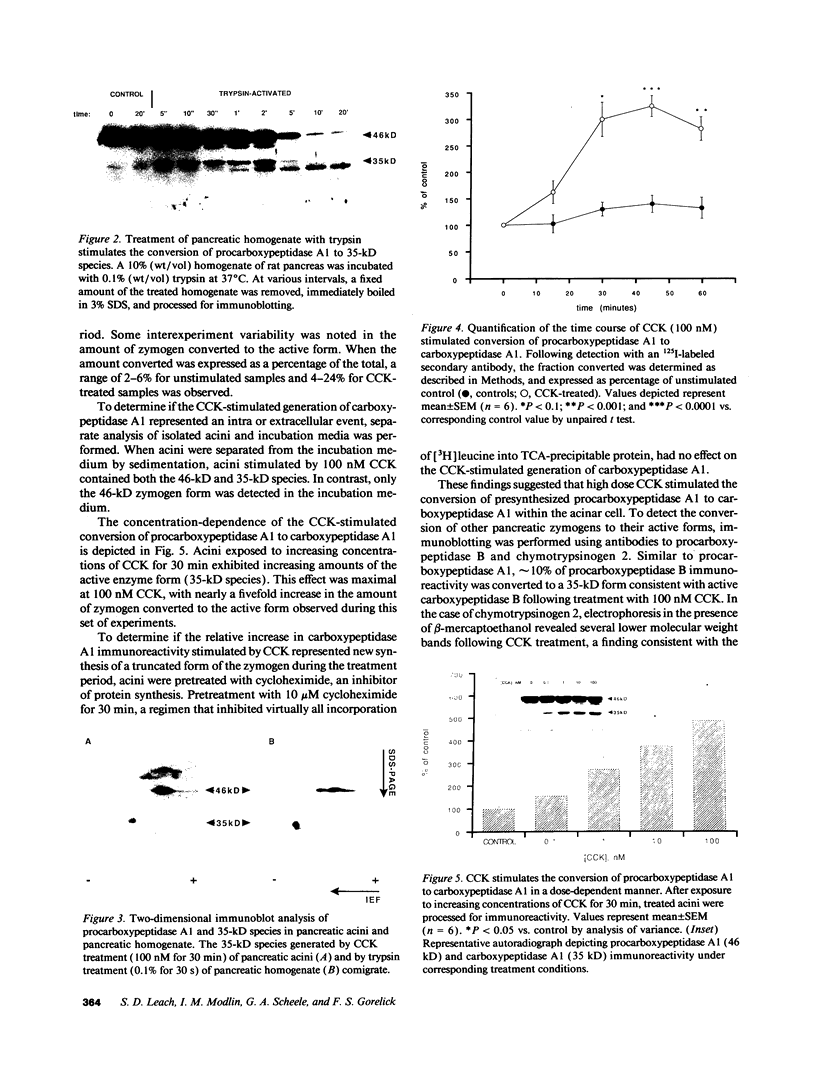
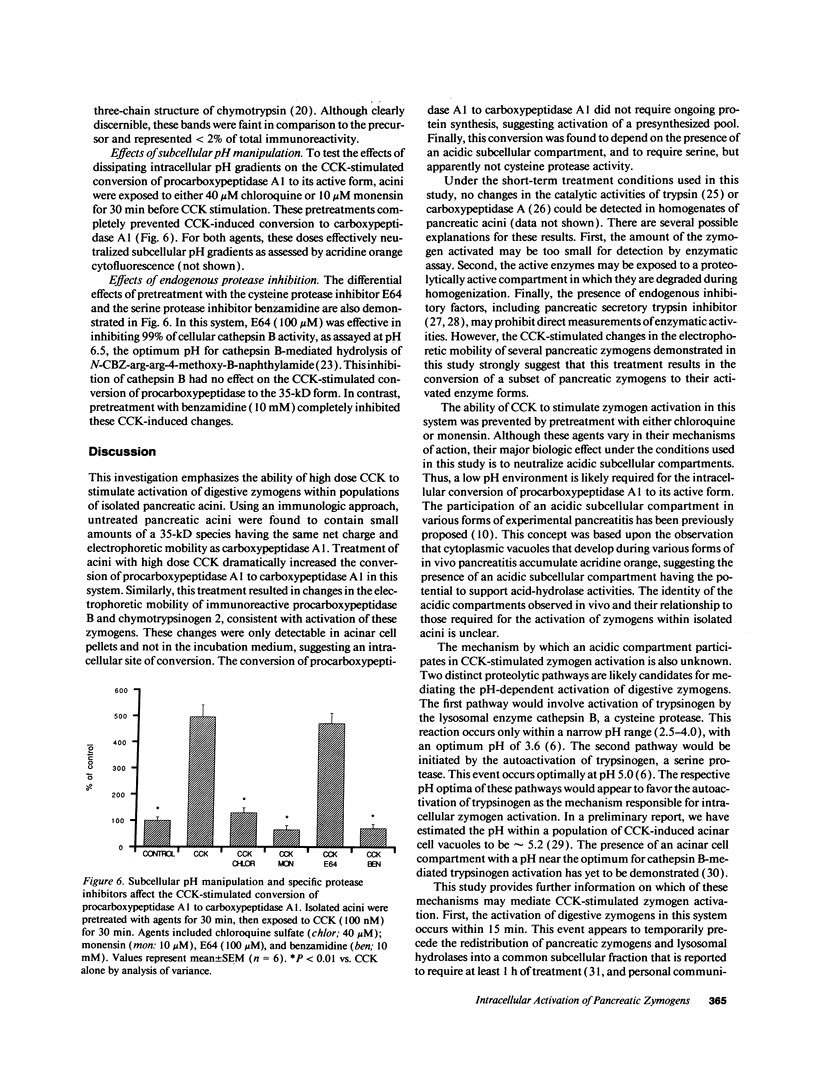
The mechanism by which digestive zymogens become activated during acute pancreatitis remains poorly understood. Given the ability for cholecystokinin (CCK) to induce pancreatitis in vivo, the effects of high dose CCK on preparations of isolated pancreatic acini were examined. Using an immunologic technique for the detection of zymogen activation, CCK was found to stimulate the conversion of procarboxypeptidase A1 to a 35-kD form having the same net charge and electrophoretic mobility as purified recombinant carboxypeptidase A1. This enhanced conversion was proportional to the dose of CCK (maximal at 100 nM), and time dependent. CCK also produced changes in the electrophoretic mobility of procarboxypeptidase B and chymotrypsinogen 2 immunoreactivity, consistent with activation of these zymogens. These events were detectable only within acinar cell pellets and not in the incubation medium, suggesting an intracellular site of conversion. The conversion of procarboxypeptidase A1 to its active form was inhibited by pretreatment with the weak base chloroquine (40 microM) and the protonophore monensin (10 microM). This conversion was also inhibited by pretreatment with the serine protease inhibitor benzamidine (10 mM) but not the cysteine protease inhibitor E64 (100 microM). The results suggest that high dose CCK stimulates the intracellular activation of digestive zymogens within isolated pancreatic acini. This event appears to require an acidic subcellular compartment and serine protease activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. M., Roman D. P., Jr, Bing D. H. Inhibition of four human serine proteases by substituted benzamidines. J Med Chem. 1978 Dec;21(12):1202–1207. doi: 10.1021/jm00210a006. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne T., Scheele G. Amino acid sequences of transport peptides associated with canine exocrine pancreatic proteins. J Biol Chem. 1982 Apr 25;257(8):4133–4140. [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Figarella C., Miszczuk-Jamska B., Barrett A. J. Possible lysosomal activation of pancreatic zymogens. Activation of both human trypsinogens by cathepsin B and spontaneous acid. Activation of human trypsinogen 1. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):293–298. [PubMed] [Google Scholar]
- GREENBAUM L. M., HIRSHKOWITZ A., SHOICHET I. The activation of trypsinogen by cathepsin B. J Biol Chem. 1959 Nov;234:2885–2890. [PubMed] [Google Scholar]
- Gardell S. J., Craik C. S., Clauser E., Goldsmith E. J., Stewart C. B., Graf M., Rutter W. J. A novel rat carboxypeptidase, CPA2: characterization, molecular cloning, and evolutionary implications on substrate specificity in the carboxypeptidase gene family. J Biol Chem. 1988 Nov 25;263(33):17828–17836. [PubMed] [Google Scholar]
- Geokas M. C., Rinderknecht H. Free proteolytic enzymes in pancreatic juice of patients with acute pancreatitis. Am J Dig Dis. 1974 Jul;19(7):591–598. doi: 10.1007/BF01073012. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal Biochem. 1982 Aug;124(2):396–405. doi: 10.1016/0003-2697(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Grinde B. Selective inhibition of lysosomal protein degradation by the thiol proteinase inhibitors E-64, Ep-459 and Ep-457 in isolated rat hepatocytes. Biochim Biophys Acta. 1982 Mar 4;701(3):328–333. doi: 10.1016/0167-4838(82)90235-7. [DOI] [PubMed] [Google Scholar]
- Kassell B., Kay J. Zymogens of proteolytic enzymes. Science. 1973 Jun 8;180(4090):1022–1027. doi: 10.1126/science.180.4090.1022. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Marks W. H., Ohlsson K. Isolation and partial characterization of the pancreatic secretory trypsin inhibitor in the rat. Biochim Biophys Acta. 1982 Jul 16;717(1):91–97. doi: 10.1016/0304-4165(82)90384-1. [DOI] [PubMed] [Google Scholar]
- McDonald J. K., Ellis S. On the substrate specificity of cathepsins B1 and B2 including a new fluorogenic substrate for cathepsin B1. Life Sci. 1975 Oct 15;17(8):1269–1276. doi: 10.1016/0024-3205(75)90137-x. [DOI] [PubMed] [Google Scholar]
- Niederau C., Grendell J. H. Intracellular vacuoles in experimental acute pancreatitis in rats and mice are an acidified compartment. J Clin Invest. 1988 Jan;81(1):229–236. doi: 10.1172/JCI113300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Anderson R. G. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature. 1987 Mar 5;326(6108):77–79. doi: 10.1038/326077a0. [DOI] [PubMed] [Google Scholar]
- Peterson L. M., Holmquist B., Bethune J. L. A unique activity assay for carboxypeptidase A in human serum. Anal Biochem. 1982 Sep 15;125(2):420–426. doi: 10.1016/0003-2697(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Rao K. N., Tuma J., Lombardi B. Acute hemorrhagic pancreatic necrosis in mice. Intraparenchymal activation of zymogens, and other enzyme changes in pancreas and serum. Gastroenterology. 1976 May;70(5 PT1):720–726. [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Schultz G. S., Sarras M. P., Jr, Gunther G. R., Hull B. E., Alicea H. A., Gorelick F. S., Jamieson J. D. Guinea pig pancreatic acini prepared with purified collagenase. Exp Cell Res. 1980 Nov;130(1):49–62. doi: 10.1016/0014-4827(80)90041-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med. 1987 Jan 15;316(3):144–150. doi: 10.1056/NEJM198701153160306. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kimura T., Mimura K., Nawata H. Activation of proteases in cerulein-induced pancreatitis. Pancreas. 1989;4(5):565–571. doi: 10.1097/00006676-198910000-00007. [DOI] [PubMed] [Google Scholar]






