Figure 1.
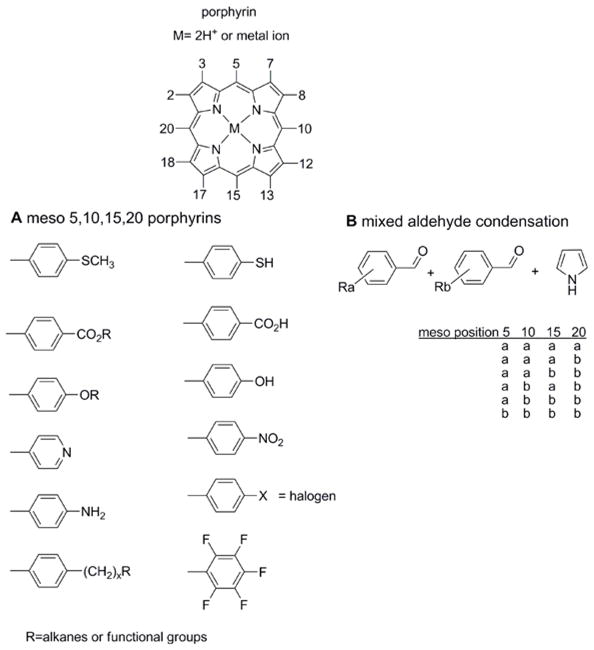
(A) Structure of the porphyrin macrocycles with a cadre of common meso (5,10,15,20) aryl derivatives. Note the meso alkane compounds are also readily accessible synthetically. (B) Much of the supramolecular chemistry of porphyrins uses less symmetric compounds, e.g. those used in the formation of SAMs on surfaces, rely on a mixed aldehyde synthesis wherein two aryl aldehydes are mixed with pyrrole to form a ‘combinatorial’ library of six compounds. The chromatographic separation of the compounds and isomers yields compounds that can be used to study molecular topologies, surface binding geometries, self-assembly into discrete arrays, or self-organization into films. Many of these compounds can also be made by more direct routes.
