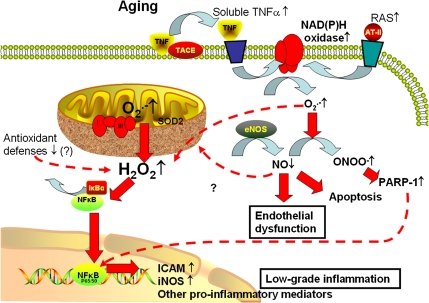
Figure 1.
Proposed scheme for pathways contributing to cellular oxidative stress and NF-κB activation in aged endothelial cells. In aged endothelial cells, increased levels of O2·− generated by the electron transport chain are dismutated to H2O2, which can penetrate the mitochondrial membrane increasing cytoplasmic H2O2 levels. H2O2 contributes to the activation of NF-κB, resulting in a proinflammatory shift in endothelial gene expression profile. Aging is also associated with upregulated expression of membrane-bound tumor necrosis factor-alpha (TNFα), which increases soluble TNFα levels in the vascular wall due to the action of TNFα-converting enzyme (TACE). In aged endothelial cells, increased levels of O2·− generated by NAD(P)H oxidases (stimulated by elevated TNFα levels and/or by the activated local renin–angiotensin system [RAS] in the vascular wall) decrease the bioavailability of NO by forming ONOO −. Lack of NO leads to vasodilator dysfunction and promotes endothelial apoptosis, whereas nitrative stress and increased H2O2 levels lead to poly(ADP-ribose) polymerase (PARP)-1 activation, which contributes to NF-κB-dependent gene transcription. Increased oxidative stress and chronic low-grade vascular inflammation increase the risk for the development of vascular diseases in the elderly patients.