Abstract
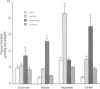
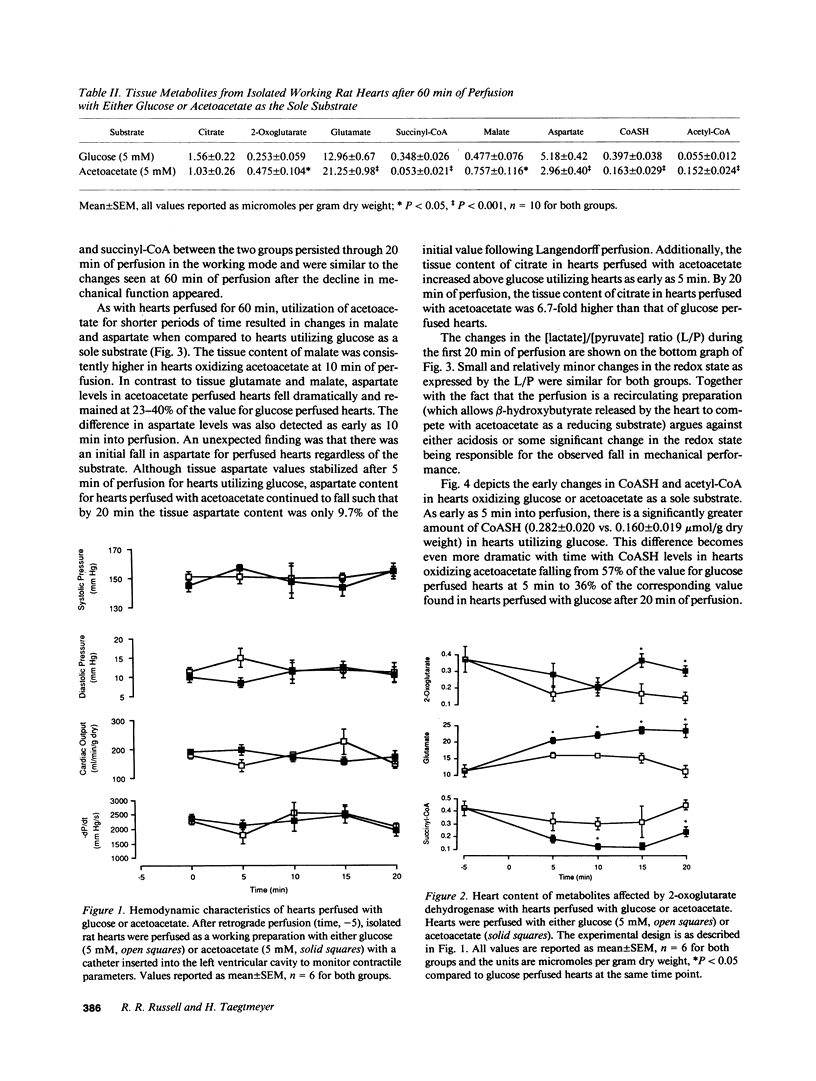
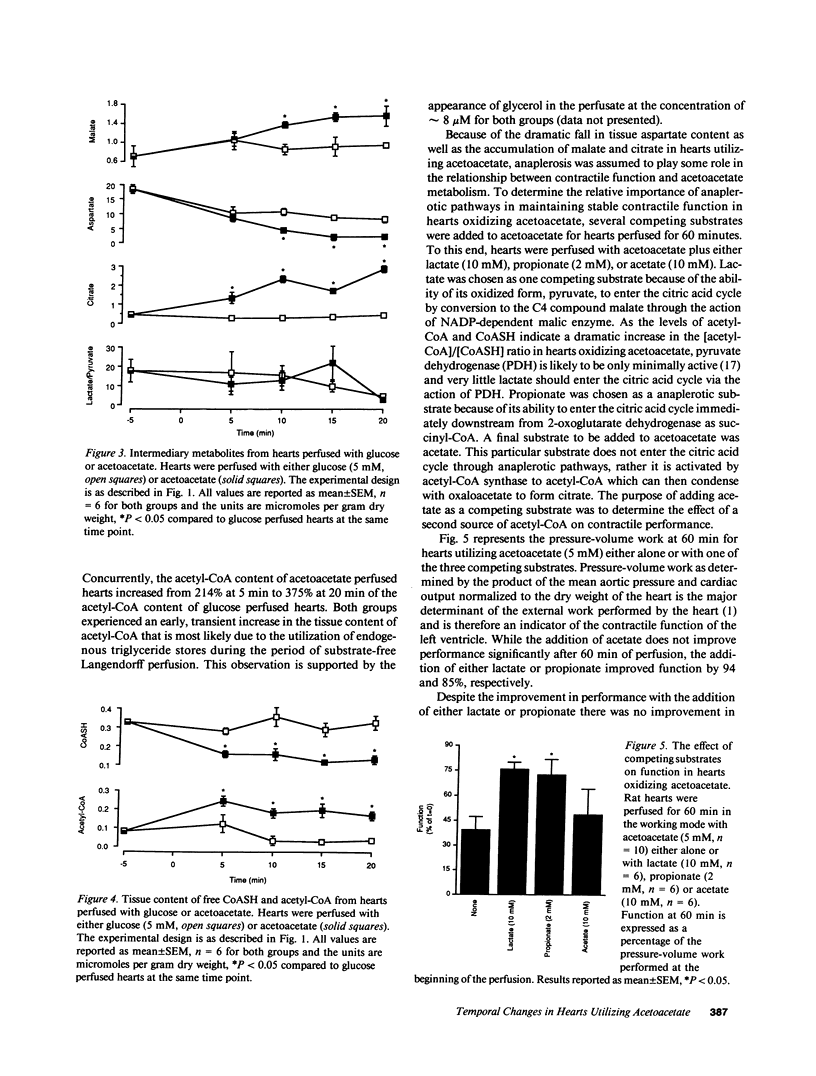
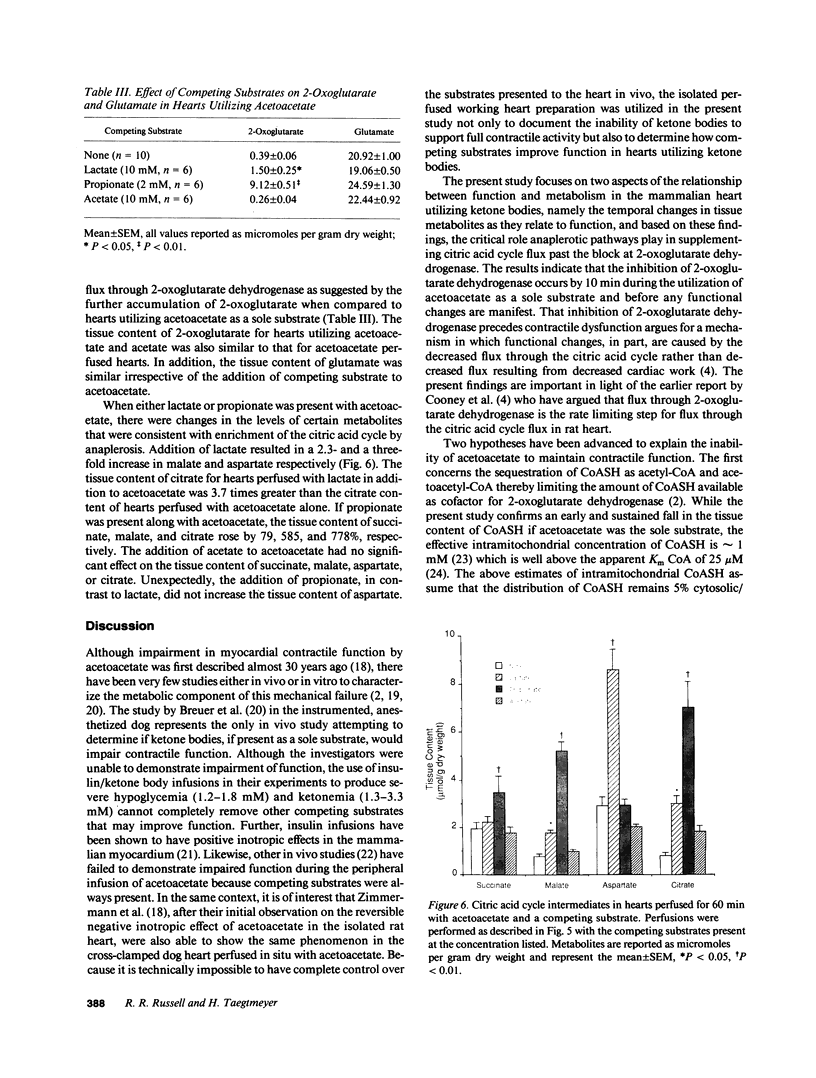
To determine the temporal relationship between changes in contractile performance and flux through the citric acid cycle in hearts oxidizing acetoacetate, we perfused isolated working rat hearts with either glucose or acetoacetate (both 5 mM) and freeze-clamped the tissue at defined times. After 60 min of perfusion, hearts utilizing acetoacetate exhibited lower systolic and diastolic pressures and lower cardiac outputs. The oxidation of acetoacetate increased the tissue content of 2-oxoglutarate and glutamate and decreased the content of succinyl-CoA suggesting inhibition of citric acid cycle flux through 2-oxoglutarate dehydrogenase. Whereas hearts perfused with either acetoacetate or glucose were similar with respect to their function for the first 20 min, changes in tissue metabolites were already observed within 5 min of perfusion at near-physiological workloads. The addition of lactate or propionate, but not acetate, to hearts oxidizing acetoacetate improved contractile performance, although inhibition of 2-oxoglutarate dehydrogenase was probably not diminished. If lactate or propionate were added, malate and citrate accumulated indicating utilization of anaplerotic pathways for the citric acid cycle. We conclude that a decreased rate of flux through 2-oxoglutarate dehydrogenase in hearts oxidizing acetoacetate precedes, and may be responsible for, contractile failure and is not the result of decreased cardiac work. Further, anaplerosis play an important role in the maintenance of contractile function in hearts utilizing acetoacetate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSENGE E., WENDT V. E., SCHOLLMEYER P., BLUEMCHEN G., GUDBJARNASON S., BING R. J. EFFECT OF KETONE BODIES ON CARDIAC METABOLISM. Am J Physiol. 1965 Jan;208:162–168. doi: 10.1152/ajplegacy.1965.208.1.162. [DOI] [PubMed] [Google Scholar]
- Bruer J., Chung K. J., Pesonen E., Haas R. H., Guth B. D., Sahn D. J., Hesselink J. R. Ketone bodies maintain normal cardiac function and myocardial high energy phosphates during insulin-induced hypoglycemia in vivo. Basic Res Cardiol. 1989 Sep-Oct;84(5):510–523. doi: 10.1007/BF01908203. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Taegtmeyer H., Newsholme E. A. Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J. 1981 Dec 15;200(3):701–703. doi: 10.1042/bj2000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. J., Spydevold O., Bremer J. Pyruvate carboxylase and propionyl-CoA carboxylase as anaplerotic enzymes in skeletal muscle mitochondria. Eur J Biochem. 1980 Sep;110(1):255–262. doi: 10.1111/j.1432-1033.1980.tb04863.x. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Lucchesi B. R., Medina M., Kniffen F. J. The positive inotropic action of insulin in the canine heart. Eur J Pharmacol. 1972 Apr;18(1):107–115. doi: 10.1016/0014-2999(72)90137-9. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn C. L., Ottaway J. H. Studies on the mechanism and kinetics of the 2-oxoglutarate dehydrogenase system from pig heart. Biochem J. 1977 Mar 1;161(3):569–581. doi: 10.1042/bj1610569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. S., Dennis S. C., DeBuysere M. S., Padma A. The regulation of pyruvate dehydrogenase in the isolated perfused rat heart. J Biol Chem. 1978 Oct 25;253(20):7369–7375. [PubMed] [Google Scholar]
- Opie L. H., Owen P. Effects of increased mechanical work by isolated perfused rat heart during production or uptake of ketone bodies. Assessment of mitochondrial oxidized to reduced free nicotinamide-adenine dinucleotide ratios and oxaloacetate concentrations. Biochem J. 1975 Jun;148(3):403–415. doi: 10.1042/bj1480403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway J. H., McClellan J. A., Saunderson C. L. Succinic thiokinase and metabolic control. Int J Biochem. 1981;13(4):401–410. doi: 10.1016/0020-711x(81)90111-7. [DOI] [PubMed] [Google Scholar]
- Ottaway J. H., McMinn C. L. The regulation of acetoacetate metabolism in heart [proceedings]. Biochem Soc Trans. 1979 Apr;7(2):411–412. doi: 10.1042/bst0070411. [DOI] [PubMed] [Google Scholar]
- Ottaway J. H., McMinn C. L. The regulation of acetoacetate metabolism in heart [proceedings]. Biochem Soc Trans. 1979 Apr;7(2):411–412. doi: 10.1042/bst0070411. [DOI] [PubMed] [Google Scholar]
- Peuhkurinen K. J. Accumulation and disposal of tricarboxylic acid cycle intermediates during propionate oxidation in the isolated perfused rat heart. Biochim Biophys Acta. 1982 Oct 11;721(2):124–134. doi: 10.1016/0167-4889(82)90060-x. [DOI] [PubMed] [Google Scholar]
- Peuhkurinen K. J., Hassinen I. E. Pyruvate carboxylation as an anaplerotic mechanism in the isolated perfused rat heart. Biochem J. 1982 Jan 15;202(1):67–76. doi: 10.1042/bj2020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G., Crabtree B., Smith G. H. The activities of 2-oxoglutarate dehydrogenase and pyruvate dehydrogenase in hearts and mammary glands from ruminants and non-ruminants. Biochem J. 1977 May 15;164(2):349–355. doi: 10.1042/bj1640349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. M., Bryla J., Williamson J. R. Regulation of mitochondrial alpha-ketoglutarate metabolism by product inhibition at alpha-ketoglutarate dehydrogenase. J Biol Chem. 1974 Mar 10;249(5):1497–1505. [PubMed] [Google Scholar]
- Sundqvist K. E., Heikkilä J., Hassinen I. E., Hiltunen J. K. Role of NADP+ (corrected)-linked malic enzymes as regulators of the pool size of tricarboxylic acid-cycle intermediates in the perfused rat heart. Biochem J. 1987 May 1;243(3):853–857. doi: 10.1042/bj2430853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist K. E., Hiltunen J. K., Hassinen I. E. Pyruvate carboxylation in the rat heart. Role of biotin-dependent enzymes. Biochem J. 1989 Feb 1;257(3):913–916. doi: 10.1042/bj2570913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980 Mar 15;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H. On the inability of ketone bodies to serve as the only energy providing substrate for rat heart at physiological work load. Basic Res Cardiol. 1983 Jul-Aug;78(4):435–450. doi: 10.1007/BF02070167. [DOI] [PubMed] [Google Scholar]
- Tahiliani A. G. Dependence of mitochondrial coenzyme A uptake on the membrane electrical gradient. J Biol Chem. 1989 Nov 5;264(31):18426–18432. [PubMed] [Google Scholar]
- Tahiliani A. G., Neely J. R. A transport system for coenzyme A in isolated rat heart mitochondria. J Biol Chem. 1987 Aug 25;262(24):11607–11610. [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- ZIMMERMAN A. N., MEIJLER F. L., HULSMANN W. C. The inhibitory effect of acetoacetate on myocardial contraction. Lancet. 1962 Oct 13;2(7259):757–758. doi: 10.1016/s0140-6736(62)90577-9. [DOI] [PubMed] [Google Scholar]