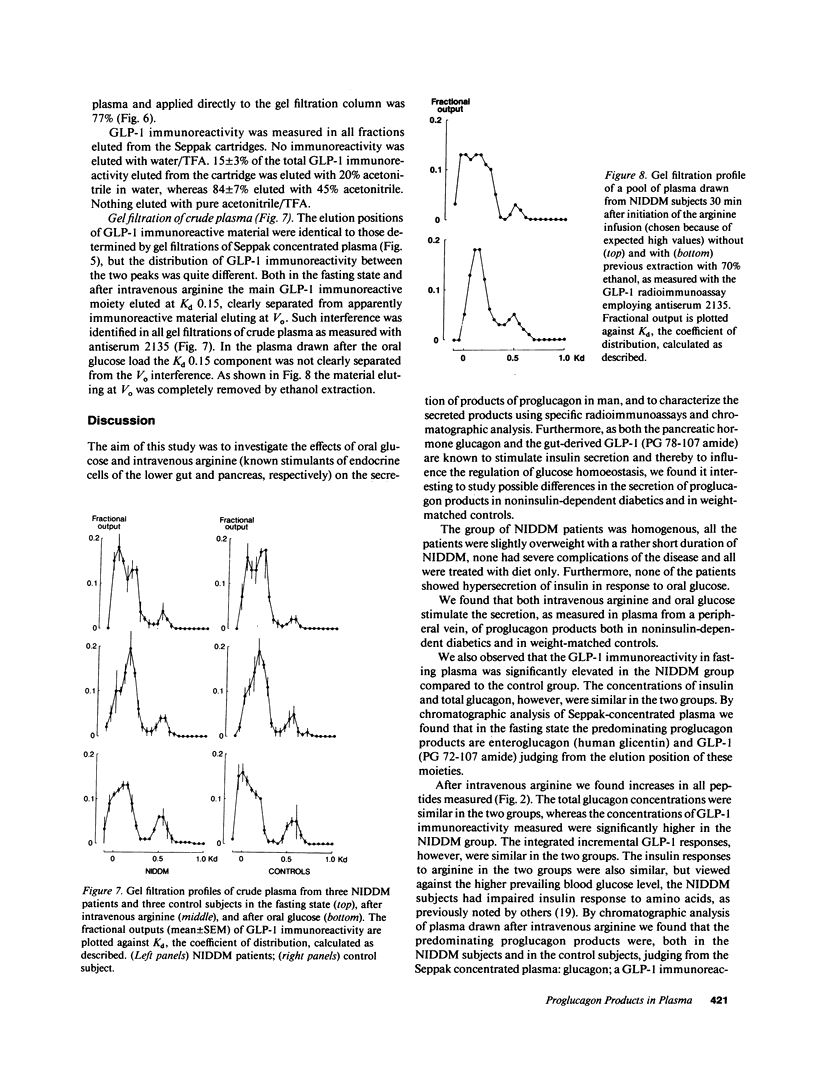
Abstract
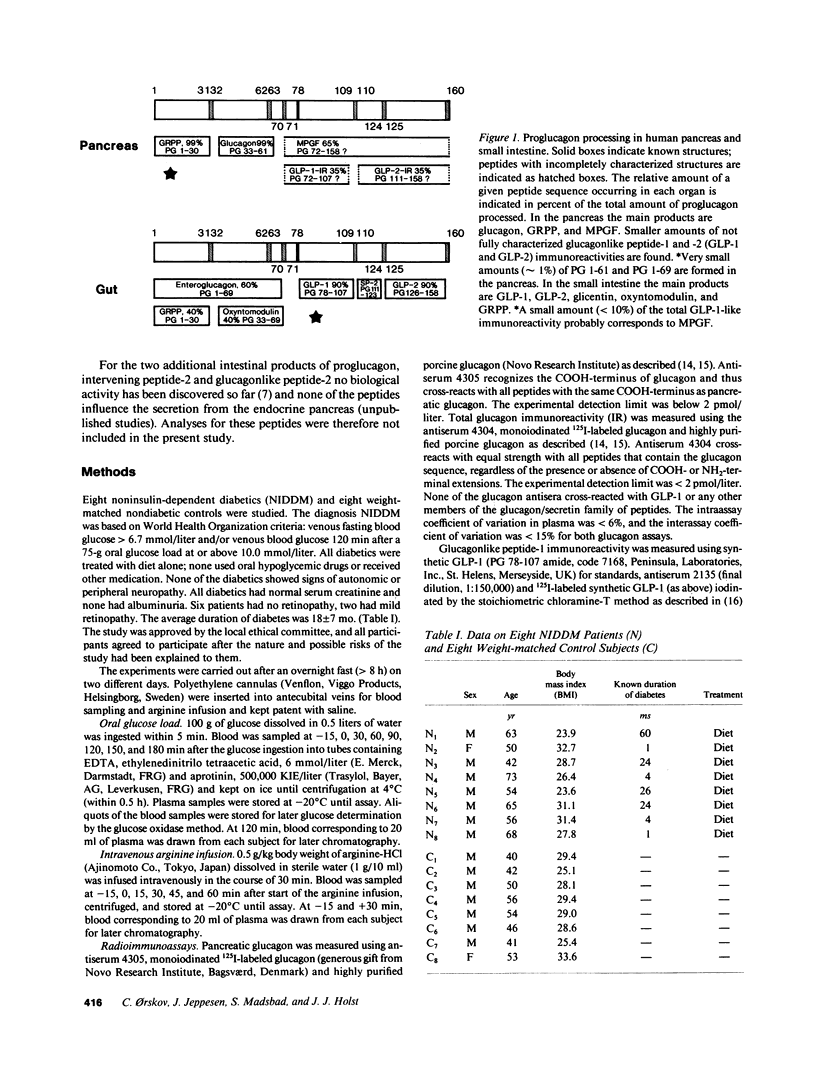
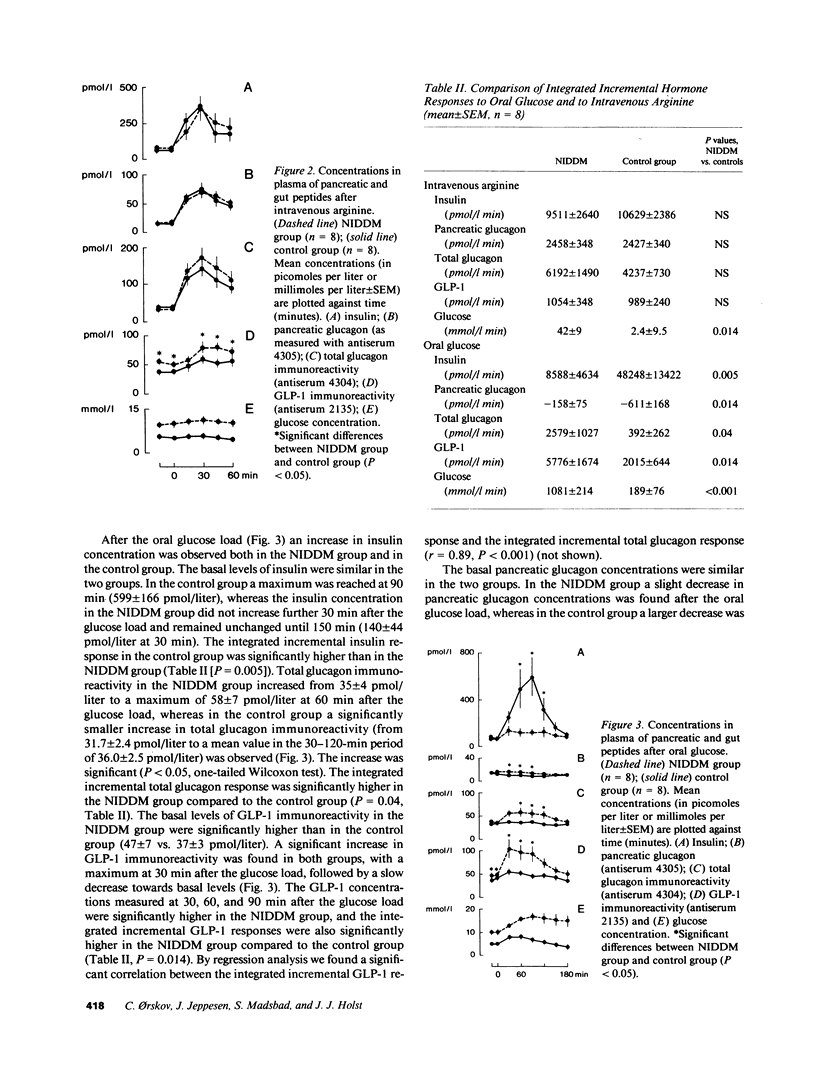
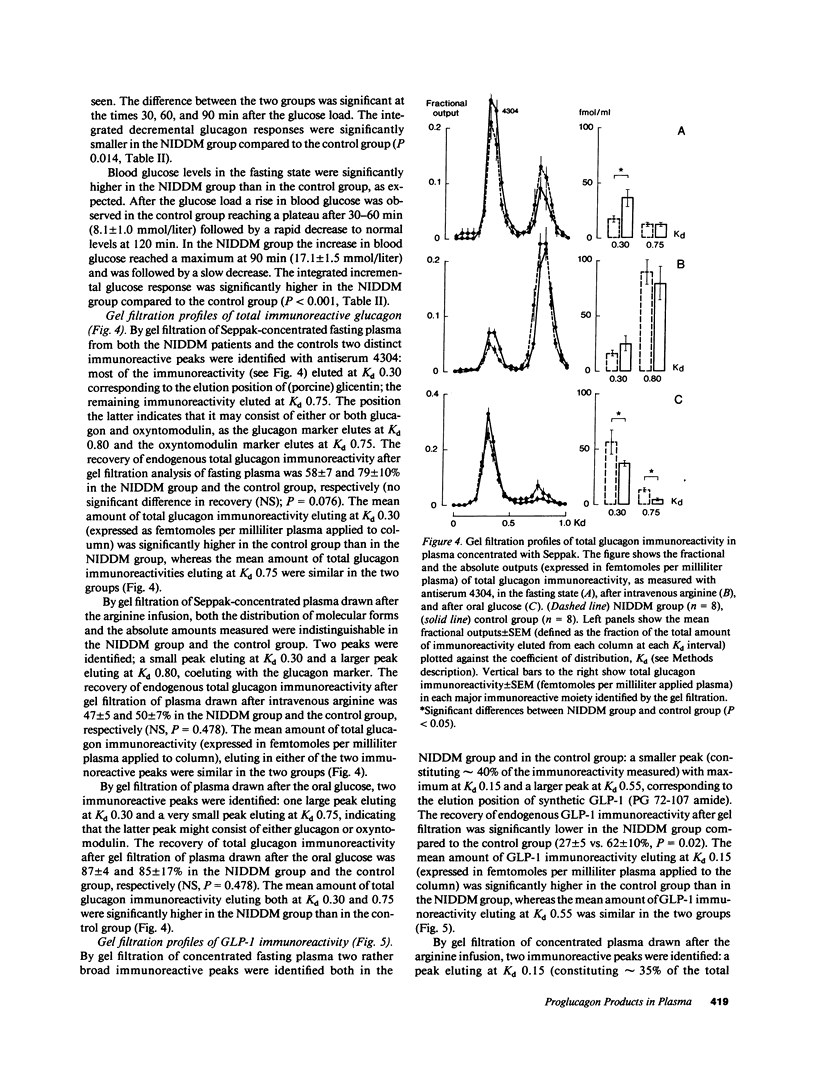
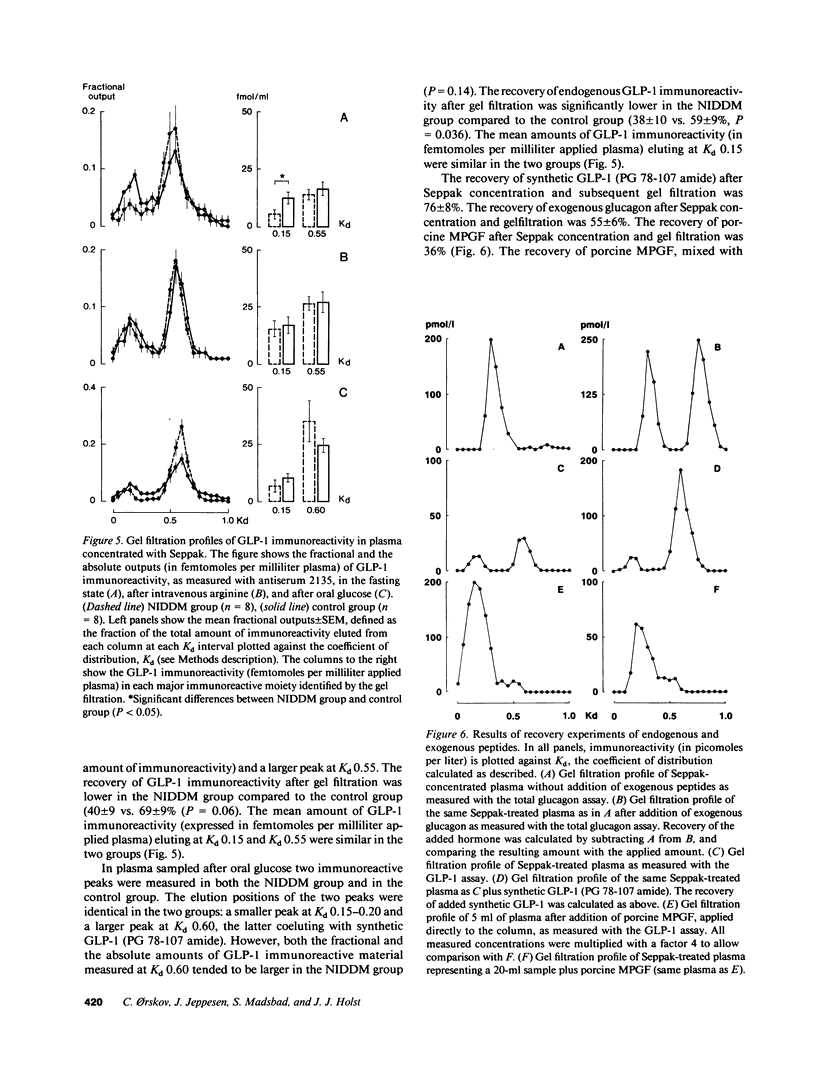
We investigated the major products of proglucagon (PG) processing in plasma in the fasting state, after intravenous arginine and after an oral glucose load in noninsulin-dependent diabetics (NIDDM) and in weight matched controls using specific radioimmunoassays and analytical gel filtration. In the fasting state the glucagonlike peptide-1 (GLP-1) immunoreactivity was significantly elevated in the NIDDM group compared with the control group. Both after intravenous arginine and after an oral glucose load a rise in the plasma concentrations of all immunoreactive moieties measured was seen. All integrated incremental responses after intravenous arginine were identical in the two groups. After oral glucose the insulin concentrations in plasma were lower and the concentrations of all proglucagon products were higher in the NIDDM group compared to the control group. The gel filtration analysis showed that arginine stimulated the secretion of pancreatic glucagon (PG 33-61), major proglucagon fragment (PG 72-158) and probably GLP-1 (PG 72-107 amide) in both groups, whereas oral glucose stimulated the secretion of glicentin (PG 1-69) and intestinal GLP-1 (PG 78-107 amide), an insulinotropic hormone. The elevated levels of immunoreactive GLP-1 in diabetics in the fasting state were mainly due to an increased concentration of major proglucagon fragment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Buhl T., Thim L., Kofod H., Orskov C., Harling H., Holst J. J. Naturally occurring products of proglucagon 111-160 in the porcine and human small intestine. J Biol Chem. 1988 Jun 25;263(18):8621–8624. [PubMed] [Google Scholar]
- George S. K., Uttenthal L. O., Ghiglione M., Bloom S. R. Molecular forms of glucagon-like peptides in man. FEBS Lett. 1985 Nov 18;192(2):275–278. doi: 10.1016/0014-5793(85)80124-1. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of pancreatic and gut glucagon in plasma. Diabetologia. 1971 Feb;7(1):10–19. doi: 10.1007/BF02346248. [DOI] [PubMed] [Google Scholar]
- Holst J. J. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem J. 1982 Dec 1;207(3):381–388. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J. Evidence that glicentin contains the entire sequence of glucagon. Biochem J. 1980 May 1;187(2):337–343. doi: 10.1042/bj1870337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J., Orskov C., Nielsen O. V., Schwartz T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987 Jan 26;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M. A., Bloom S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987 Dec 5;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- Kåresen R., Tronier B., Aune S. Immunoreactive glucagon and insulin C-peptide in man after resection of the pancreas and total pancreatectomy. Am J Surg. 1980 Aug;140(2):272–276. doi: 10.1016/0002-9610(80)90021-5. [DOI] [PubMed] [Google Scholar]
- Mojsov S., Weir G. C., Habener J. F. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987 Feb;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C., Bersani M., Johnsen A. H., Højrup P., Holst J. J. Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J Biol Chem. 1989 Aug 5;264(22):12826–12829. [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Knuhtsen S., Baldissera F. G., Poulsen S. S., Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986 Oct;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Nielsen O. V. Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology. 1988 Oct;123(4):2009–2013. doi: 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Poulsen S. S., Kirkegaard P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia. 1987 Nov;30(11):874–881. doi: 10.1007/BF00274797. [DOI] [PubMed] [Google Scholar]
- Orskov C., Holst J. J. Radio-immunoassays for glucagon-like peptides 1 and 2 (GLP-1 and GLP-2). Scand J Clin Lab Invest. 1987 Apr;47(2):165–174. [PubMed] [Google Scholar]
- Patzelt C., Schiltz E. Conversion of proglucagon in pancreatic alpha cells: the major endproducts are glucagon and a single peptide, the major proglucagon fragment, that contains two glucagon-like sequences. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5007–5011. doi: 10.1073/pnas.81.16.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L., Moody A. J. The primary structure of porcine glicentin (proglucagon). Regul Pept. 1981 May;2(2):139–150. doi: 10.1016/0167-0115(81)90007-0. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. K., Bolgiano D. C., McKnight B., Halter J. B., Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984 Oct;74(4):1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C., Mojsov S., Hendrick G. K., Habener J. F. Glucagonlike peptide I (7-37) actions on endocrine pancreas. Diabetes. 1989 Mar;38(3):338–342. doi: 10.2337/diab.38.3.338. [DOI] [PubMed] [Google Scholar]


