Abstract
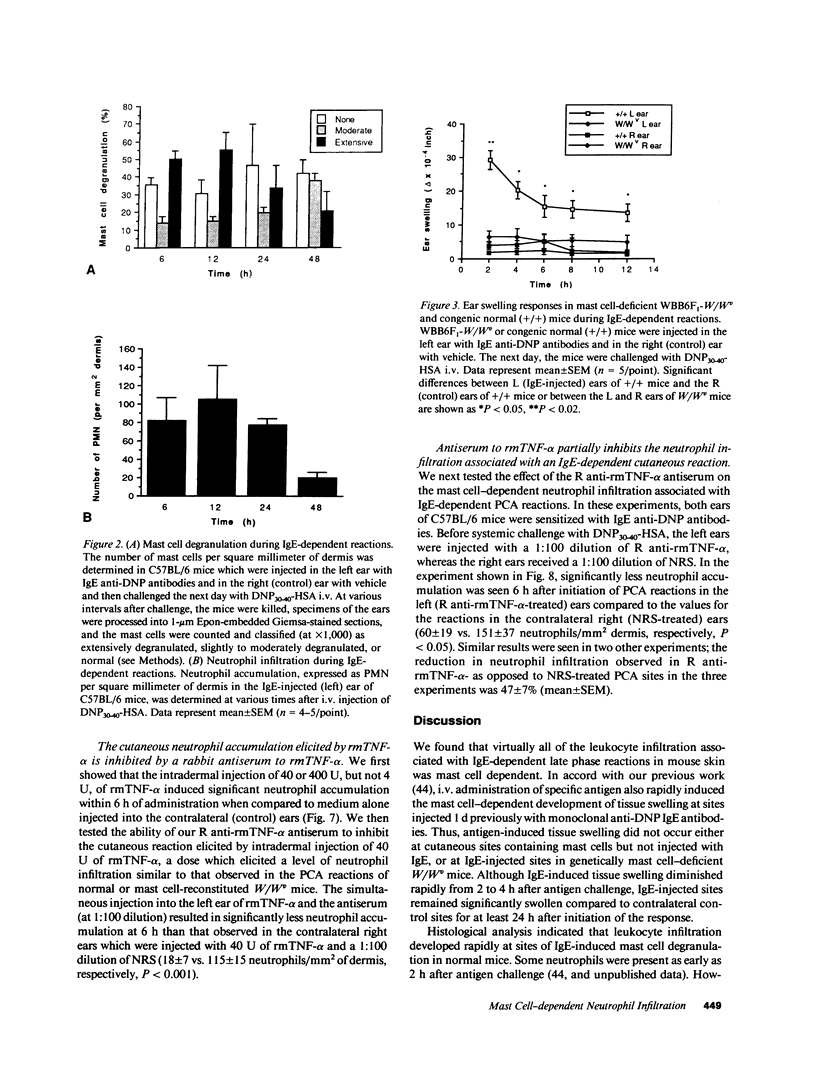
Much of the clinically important pathology associated with IgE-dependent disorders is thought to reflect the actions of the blood-borne leukocytes recruited during these responses. To evaluate the extent to which mast cells are responsible for the leukocyte infiltration associated with IgE-dependent cutaneous reactions, we attempted to elicit these responses in normal mice, genetically mast cell-deficient W/Wv mice, and in W/Wv mice selectively repaired of their mast cell deficiency by the intradermal injection of cultured mast cells derived from the congenic normal (+/+) mice. We found that the tissue swelling associated with IgE-dependent passive cutaneous anaphylaxis reactions developed rapidly and diminished markedly from 2 to 4 h after antigen challenge, but remained detectable for at least 24 h after elicitation of the responses. Infiltration of leukocytes (predominantly neutrophils) also occurred at these sites, but reached maximal levels 6-12 h after antigen challenge, persisted at high levels for 24 h, and largely waned by 48 h. Virtually all of the tissue swelling and leukocyte infiltration associated with IgE-dependent cutaneous reactions was mast cell dependent. Intradermal injection of 40 U of recombinant murine TNF-alpha (rmTNF-alpha) elicited neutrophil infiltration similar in magnitude and kinetics to that observed after IgE-dependent mast cell degranulation. A rabbit anti-rmTNF-alpha (R anti-rmTNF-alpha) antiserum, which was able to inhibit 84% of the neutrophil infiltration observed after i.d. injection of rmTNF-alpha, inhibited IgE-, and mast cell-dependent leukocyte infiltration by 47 +/- 7% in three separate experiments. These findings indicate that TNF-alpha contributes to mast cell-dependent recruitment of leukocytes during IgE-dependent cutaneous late phase reactions, but suggest that other mast cell-associated mediators probably also contribute to this response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd P. R., Rollins B. J., Wilson S. D., Billings P. R., Stiles C. D., Dorf M. E. Comparison of fibroblast and T-cell activation genes. Cell Immunol. 1988 Sep;115(2):481–483. doi: 10.1016/0008-8749(88)90200-6. [DOI] [PubMed] [Google Scholar]
- Capron A., Capron M., Grangette C., Dessaint J. P. IgE and inflammatory cells. Ciba Found Symp. 1989;147:153–170. doi: 10.1002/9780470513866.ch10. [DOI] [PubMed] [Google Scholar]
- Charlesworth E. N., Hood A. F., Soter N. A., Kagey-Sobotka A., Norman P. S., Lichtenstein L. M. Cutaneous late-phase response to allergen. Mediator release and inflammatory cell infiltration. J Clin Invest. 1989 May;83(5):1519–1526. doi: 10.1172/JCI114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dolovich J., Denberg J., Kwee Y. N., Belda T., Blajchman J., Hargreave F. E. Does non-immunologic mast cell mediator release/activation elicit a late cutaneous response? Ann Allergy. 1983 Apr;50(4):241–244. [PubMed] [Google Scholar]
- Dolovich J., Hargreave F. E., Chalmers R., Shier K. J., Gauldie J., Bienenstock J. Late cutaneous allergic responses in isolated IgE-dependent reactions. J Allergy Clin Immunol. 1973 Jul;52(1):38–46. doi: 10.1016/0091-6749(73)90119-x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M., Barnes B. A., Manseau E. J., Galli S. J. Rejection of first-set skin allografts in man. the microvasculature is the critical target of the immune response. J Exp Med. 1979 Aug 1;150(2):322–337. doi: 10.1084/jem.150.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Dvorak H. F. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- Galli S. J., Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984 Nov 9;226(4675):710–713. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New insights into "the riddle of the mast cells": microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990 Jan;62(1):5–33. [PubMed] [Google Scholar]
- Galli S. J., Wershil B. K., Gordon J. R., Martin T. R. Mast cells: immunologically specific effectors and potential sources of multiple cytokines during IgE-dependent responses. Ciba Found Symp. 1989;147:53–73. doi: 10.1002/9780470513866.ch5. [DOI] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Ghiara P., Boraschi D., Villa L., Scapigliati G., Taddei C., Tagliabue A. In vitro generated mast cells express natural cytotoxicity against tumour cells. Immunology. 1985 Jun;55(2):317–324. [PMC free article] [PubMed] [Google Scholar]
- Gordon J. R., Burd P. R., Galli S. J. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990 Dec;11(12):458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Granstein R. D., Margolis R., Mizel S. B., Sauder D. N. In vivo inflammatory activity of epidermal cell-derived thymocyte activating factor and recombinant interleukin 1 in the mouse. J Clin Invest. 1986 Mar;77(3):1020–1027. doi: 10.1172/JCI112354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D., Crowle P. K. Immune response potential of mast cell-deficient W/Wv mice. Int Arch Allergy Appl Immunol. 1986;80(1):85–94. doi: 10.1159/000234031. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D. Systemic anaphylaxis in mast-cell-deficient mice of W/Wv and Sl/Sld genotypes. Exp Cell Biol. 1987;55(2):63–68. doi: 10.1159/000163399. [DOI] [PubMed] [Google Scholar]
- Jacoby W., Cammarata P. V., Findlay S., Pincus S. H. Anaphylaxis in mast cell-deficient mice. J Invest Dermatol. 1984 Oct;83(4):302–304. doi: 10.1111/1523-1747.ep12340431. [DOI] [PubMed] [Google Scholar]
- Jadus M. R., Schmunk G., Djeu J. Y., Parkman R. Morphology and lytic mechanisms of interleukin 3-dependent natural cytotoxic cells: tumor necrosis factor as a possible mediator. J Immunol. 1986 Nov 1;137(9):2774–2783. [PubMed] [Google Scholar]
- Kaliner M., Lemanske R. Inflammatory responses to mast cell granules. Fed Proc. 1984 Oct;43(13):2846–2851. [PubMed] [Google Scholar]
- Kikutani H., Yokota A., Uchibayashi N., Yukawa K., Tanaka T., Sugiyama K., Barsumian E. L., Suemura M., Kishimoto T. Structure and function of Fc epsilon receptor II (Fc epsilon RII/CD23): a point of contact between the effector phase of allergy and B cell differentiation. Ciba Found Symp. 1989;147:23–35. doi: 10.1002/9780470513866.ch3. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Klein L. M., Lavker R. M., Matis W. L., Murphy G. F. Degranulation of human mast cells induces an endothelial antigen central to leukocyte adhesion. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8972–8976. doi: 10.1073/pnas.86.22.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanske R. F., Jr, Kaliner M. Mast cell-dependent late phase reactions. Clin Immunol Rev. 1981;1(4):547–580. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Kawakita K., Kiso Y., Nakano T., Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989 Feb 1;142(3):927–931. [PubMed] [Google Scholar]
- Mekori Y. A., Chang J. C., Wershil B. K., Galli S. J. Studies of the role of mast cells in contact sensitivity responses. Passive transfer of the reaction into mast cell-deficient mice locally reconstituted with cultured mast cells: effect of reserpine on transfer of the reaction with DNP-specific cloned T cells. Cell Immunol. 1987 Oct 1;109(1):39–52. doi: 10.1016/0008-8749(87)90290-5. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Galli S. J. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol. 1985 Aug;135(2):879–885. [PubMed] [Google Scholar]
- Metzger H., Kinet J. P., Blank U., Miller L., Ra C. The receptor with high affinity for IgE. Ciba Found Symp. 1989;147:93–113. [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel H. L., Kaliner M. The biologic activity of mast cell granules. III. Purification of inflammatory factors of anaphylaxis (IF-A) responsible for causing latephase reactions. J Immunol. 1981 Oct;127(4):1398–1402. [PubMed] [Google Scholar]
- Okuno T., Takagaki Y., Pluznik D. H., Djeu J. Y. Natural cytotoxic (NC) cell activity in basophilic cells: release of NC-specific cytotoxic factor by IgE receptor triggering. J Immunol. 1986 Jun 15;136(12):4652–4658. [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Effects of tumour necrosis factor and related cytokines on vascular endothelial cells. Ciba Found Symp. 1987;131:170–184. doi: 10.1002/9780470513521.ch12. [DOI] [PubMed] [Google Scholar]
- Sayers T. J., Wiltrout T. A., Bull C. A., Denn A. C., 3rd, Pilaro A. M., Lokesh B. Effect of cytokines on polymorphonuclear neutrophil infiltration in the mouse. Prostaglandin- and leukotriene-independent induction of infiltration by IL-1 and tumor necrosis factor. J Immunol. 1988 Sep 1;141(5):1670–1677. [PubMed] [Google Scholar]
- Schleimer R. P., Fox C. C., Naclerio R. M., Plaut M., Creticos P. S., Togias A. G., Warner J. A., Kagey-Sobotka A., Lichtenstein L. M. Role of human basophils and mast cells in the pathogenesis of allergic diseases. J Allergy Clin Immunol. 1985 Aug;76(2 Pt 2):369–374. doi: 10.1016/0091-6749(85)90656-6. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Solley G. O., Gleich G. J., Jordon R. E., Schroeter A. L. The late phase of the immediate wheal and flare skin reaction. Its dependence upon IgE antibodies. J Clin Invest. 1976 Aug;58(2):408–420. doi: 10.1172/JCI108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum S., Oertel H., Henderson W., Kaliner M. The biologic activity of mast cell granules. I. Elicitation of inflammatory responses in rat skin. J Immunol. 1980 Jul;125(1):325–335. [PubMed] [Google Scholar]
- Wershil B. K., Mekori Y. A., Murakami T., Galli S. J. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987 Oct 15;139(8):2605–2614. [PubMed] [Google Scholar]
- Wershil B. K., Murakami T., Galli S. J. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988 Apr 1;140(7):2356–2360. [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Davatelis G., Sherry B., Beutler B., Hesse D. G., Nguyen H. T., Moldawer L. L., Nathan C. F., Lowry S. F., Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988 Feb 1;167(2):570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Wershil B. K., Arizono N., Galli S. J. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989 Oct;84(4):1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Liu C. C., Butler G., Cohn Z. A., Galli S. J. Identification, purification, and characterization of a mast cell-associated cytolytic factor related to tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9175–9179. doi: 10.1073/pnas.84.24.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]






