Abstract
OBJECTIVE
Antiretroviral (ARV) drugs are routinely provided to HIV-infected pregnant women to prevent HIV mother-to-child transmission. Although ARV use has significantly reduced mother-to-child transmission to <2% in the United States, it remains crucial to monitor uninfected infants and children for adverse consequences of in utero ARV exposure.
METHODS
We studied neurodevelopmental function in HIV-exposed uninfected children who were enrolled in Pediatric AIDS Clinical Trials Group 219/219C, a multisite, prospective, cohort study. Mental and motor functioning were assessed with the Bayley Scales of Infant Development (BSID), first and second editions. ARV exposure information was collected during pregnancy or within the first years of life. Linear regression methods were used to evaluate the association of in utero ARV exposure on Mental Developmental Index and Psychomotor Developmental Index at 2 years of age, controlling for demographic factors (age, gender, and race/ethnicity) and potential confounders: test version, primary language, primary caregiver, caregiver education level, low birth weight, geographic and urban/rural location, birth year, and maternal illicit drug use.
RESULTS
Among 1840 infants who were born between 1993 and 2006, 1694 (92%) were exposed to ARV in utero and 146 (8%) were not exposed. After controlling for confounders, children who were exposed in utero to any ARV did not have lower Mental Developmental Index and Psychomotor Developmental Index scores than unexposed children. Among low birth weight infants, significantly higher BSID scores were observed for prenatally ARV-exposed than unexposed children. Maternal illicit drug use was reported for 17% of mothers but was not associated with BSID scores.
CONCLUSIONS
Mental and motor functioning scores were not lower for infants with in utero ARV exposure compared with no exposure. Although these results are reassuring, continued evaluation of uninfected children with in utero ARV exposure for long-term adverse outcomes is important.
Keywords: Bayley scales, mental development, motor development, maternal health, antiretroviral treatment, low birth weight
Antiretroviral (ARV) drugs are routinely provided to HIV-infected pregnant women to prevent HIV mother-to-child transmission (MTCT) and have dramatically reduced MTCT in the United States and other developed countries.1-4 In particular, use of zidovudine (ZDV) during pregnancy, intrapartum ZDV treatment, and short-term neonatal treatment was demonstrated by the AIDS Clinical Trials Group 076 study to reduce perinatal transmission from 25% to 8%.1 Treatment with highly active antiretroviral therapy (HAART) in resource-rich countries has further reduced transmission to <2%.2-4 Perinatal HIV prevention programs are now implemented in many countries across the world, making in utero ARV exposure and its potential consequences a global issue. Since 1998, the US Public Health Service has recommended the use of combination ARV to prevent MTCT of HIV.5 Despite the clear successes of these programs in reducing transmission rates, concern remains regarding possible adverse consequences of prenatal exposure, given that many ARV drugs readily cross the placenta and some have demonstrated mutagenic and carcinogenic effects in animal studies.6-8
In response to such concerns, a number of studies have been conducted to evaluate whether congenital malformations, cancer, growth delay, neurodevelopmental problems, hematologic and lactic acid abnormalities, and potential mitochondrial toxicity could be associated with prenatal ARV exposure in infants who are born to HIV-infected mothers. These studies have generally provided reassuring support for the safety of perinatal regimens,9,10 showing little evidence of increased risk for congenital malformations,10-12 early childhood cancers,9,13,14 or growth abnormalities.15,16 Several studies have shown that in utero ARV exposure (particularly to combination ARV agents) may be associated with mild but persistent hematologic abnormalities in lymphocytes or neutrophil populations.17-20 The evidence regarding mitochondrial toxicity in HIV-exposed and ARV-exposed children remains equivocal; some studies support a lack of association of mitochondrial dysfunction with perinatal ARV exposure in uninfected infants,9-11,21-26 whereas others suggest potential associations with nucleoside reverse transcriptase inhibitors (NRTIs), particularly combinations such as ZDV plus lamivudine (3TC).27-30
Few studies have evaluated the effects of prenatal ARV exposure on neurodevelopment in uninfected children. An evaluation of children who were enrolled in the Women and Infants Transmission Study (WITS) compared declines in cognitive and motor functioning between 114 HIV-infected versus 481 HIV-exposed children and found significantly higher risk for decline in the HIV-infected children31; however, this study did not address the impact of maternal ARV exposure. An evaluation of the Pediatric AIDS Clinical Trials Group (PACTG) 219/219C cohort compared changes in neurodevelopmental functioning in HIV-infected children who were treated with protease inhibitors with those of uninfected children.32 Investigators observed no effect of prenatal ARV exposure on cognitive and motor functioning in uninfected children who were younger than 1 year but did not adjust for any potential confounders. One study examined the effect of in utero exposure to HAART on cognitive functioning of HIV-uninfected children.33 A significant difference was observed in mean mental development scores in 39 HIV-uninfected children who were exposed to HAART compared with 24 control children from an ongoing hepatitis C study; however, after adjustment for maternal substance use, the difference in average mental scores was not statistically significant. Adjustment for other potential confounders was not possible because of the small study size.
We studied neurodevelopmental function in 1840 HIV-exposed uninfected children who were enrolled in PACTG 219/219C, a multisite, prospective, cohort study. Our assessment compared mental and motor development scores between those with and without maternal ARV exposure, controlling for many factors that are associated with cognitive functioning, including primary language, race/ethnicity, birth weight, and caregiver characteristics. We also conducted sensitivity analyses to evaluate the timing and duration of prenatal ARV exposure and specific regimens of interest and their association with neurodevelopmental function.
METHODS
This study was based on data collected as part of the PACTG 219 and 219C cohort studies, which enrolled children in the United States with HIV infection or perinatal HIV exposure between 1993 and 2006. PACTG 219 was initiated to study long-term effects of in utero ARV exposure and complications of HIV infection in children who were co-enrolled in another PACTG treatment trial or whose mother participated in a PACTG perinatal treatment trial. A revised version, PACTG 219C, opened in 2000 and extended enrollment to any youth who was aged ≤21 and had HIV infection or perinatal HIV exposure. Children and adolescents were enrolled at >80 participating sites across the United States, including Puerto Rico. The study protocol was reviewed and approved by the institutional review board at each participating site, and written informed consent was obtained from each child's parent/guardian. The study closed to follow-up in May 2007; additional details of study conduct have been previously reported.34,35
Our analysis focused on children who were perinatally HIV-exposed but uninfected and who had at least 1 neurodevelopmental functioning test by using the Bayley Scales of Infant Development (BSID). At the time of enrollment into PACTG 219/219C, clinical records were abstracted to obtain medical and clinical histories. ARV exposure information was collected during pregnancy or within the first years of life (75% enrolled before age 1 and 90% before age 18 months) and was supplemented with data collected in other PACTG studies (primarily perinatal protocols 076, 185, and 316). Maternal HIV RNA levels during pregnancy were not collected in 219/219C but were obtained from these perinatal protocols when available.
Neurodevelopmental functioning in children up to 3 years of age was assessed by using the BSID I36; the BSID II replaced the BSID I study-wide in March 1996, after being revised by test developers to address both changes in theory and developmental research and the upward shifts in scores.37,38 BSID were scheduled to be administered as study-required assessments by trained psychologists according to standardized procedures every 6 months until 36 months of age in PACTG 219 and at 6, 12, 24, and 36 months of age in PACTG 219C. Our analysis included the BSID test conducted closest to 24 months of age. Floor-adjusted scaled scores were used, and results that were judged invalid by the site psychologist and confirmed invalid after review by team psychologists were excluded. The Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI) were used to measure general functioning in mental and motor skills, respectively. The MDI and PDI are well-established measures that are standardized to have a mean 100 and an SD 16 (BSID I)36 or 15 (BSID II).37 Our study of 1840 individuals had 90% power to detect differences in BSID test scores of ≥4.2 points between exposed and unexposed subjects.
Linear regression methods were used to estimate the association of in utero ARV exposure on MDI and PDI scores, controlling for demographic factors (age at test, gender, and race/ethnicity) and potential confounders: test version (BSID I or II), primary language, primary caregiver, caregiver education level, low birth weight (LBW; >2500 g), and birth year. In addition, we controlled for variation on the basis of the size of clinical unit (patient accrual), geographic location in the United States, and urban/rural location. The effects of ARV and other covariates on MDI and PDI scores are summarized by using least squares means; these adjusted means reflect the predicted BSID scores when all other model covariates were set to their average values. Interactions between in utero ARV exposure and other covariates were explored to evaluate possible effect modification. Because of the reported decrease in mean scores for the BSID II as compared with the BSID I test versions,38 along with the coincident introduction of pro-tease inhibitors,39 we also conducted a stratified analysis by test version. In addition, subgroup analyses were conducted among the subset of women with information available on maternal substance use (heroin, cocaine, or other stimulants, n = 1162) and among those with maternal viral load measurements (n = 936) during pregnancy.
Comparisons were also conducted of the proportion of children in each group with severe mental or motor impairment, defined as an MDI or PDI score <70, respectively, both overall and adjusted for potential confounders by using logistic regression analysis. Analyses were conducted using SAS 9 (SAS Institute Inc, Cary, NC) and included data submitted as of November 2006. All P values are 2-sided, and P < .05 was considered statistically significant.
RESULTS
A total of 2342 uninfected, HIV-exposed infants were enrolled in PACTG 219 or 219C as of November 2006, 2300 of whom were enrolled within the recommended age range for BSID I and II testing. Among these 2300 infants, 1910 had at least 1 BSID test reported, and 1840 (96%) of these had both valid test results and known maternal ARV exposure (47 were judged invalid after review by team psychologists, and 23 were excluded because of missing ARV exposure). These 1840 infants were born between 1993 and 2006 and included 1694 (92%) children who were exposed and 146 (8%) who were not exposed to ARV in utero. Demographic characteristics of the study population are summarized in Table 1. Of the 1840 children, 293 (16%) had LBW. Maternal use of heroin, cocaine, or stimulants during pregnancy was self-reported by 17% of those with information available. Although most characteristics were similar by exposure status, those with in utero ARV exposure were more likely to be born after 1994 (89% vs 22%) and to have been assessed by using the BSID II test (81% vs 21%). There was no significant difference in background characteristics for those who were excluded because of invalid BSID scores, but the 23 who were excluded because of missing ARV exposure were less likely to have a biological parent as caregiver.
TABLE 1.
Demographic, Health, and Site Characteristics of 1840 HIV-Exposed Uninfected Study Participants Overall and by In Utero ARV Exposure
| Characteristic | All Study Subjects (N= 1840) |
In Utero ARV Exposure |
|
|---|---|---|---|
| Exposed (n = 1694) |
Not Exposed (n = 146) |
||
| Female, n (%) | 921 (50) | 844 (50) | 77 (53) |
| Age at test, median (IQR) | 1.8 (1.0–2.0) | 1.6 (1.0–2.0) | 1.8 (1.5–2.1) |
| Race/ethnicity, n (%) | |||
| White non-Hispanic and other races | 242 (13) | 219 (13) | 23 (16) |
| Black non-Hispanic | 1016 (55) | 933 (55) | 83 (57) |
| Hispanic | 582 (32) | 542 (32) | 40 (27) |
| Non-English primary language, n (%) | 485 (26) | 454 (27) | 31 (21) |
| Biological parent as primary caregiver, n (%) | 1758 (96) | 1624 (96) | 134 (92) |
| Education level of primary caregiver, n (%) | |||
| Less than high school | 642 (35) | 589 (35) | 53 (36) |
| High school graduate | 601 (32) | 557 (33) | 44 (30) |
| Some college/technical school | 401 (22) | 365 (21) | 36 (25) |
| College graduate or higher | 93 (5) | 88 (5) | 5 (3) |
| Other/unknown | 103 (6) | 95 (6) | 8 (6) |
| LBW, n (%) | 293 (16) | 274 (16) | 19 (13) |
| BSID II used, n (%) | 1391 (76) | 1360 (81) | 31 (21) |
| Child;s year of birth, n (%) | |||
| 1991–1994 | 294 (16) | 180 (11) | 114 (78) |
| 1994–2000 | 817 (44) | 804 (47) | 13 (9) |
| 2000–2006 | 729 (40) | 710 (42) | 19 (13) |
| Small Clinical site (<45 accrued infants), n (%) | 1034 (56) | 936 (55) | 98 (67) |
| Region, n (%) | |||
| Northeast | 579 (32) | 546 (32) | 33 (23) |
| South | 851 (46) | 779 (46) | 72 (49) |
| Midwest | 165 (9) | 143 (9) | 22 (15) |
| West | 245 (13) | 226 (13) | 19 (13) |
| Urban site (>1 million population), n (%) | 1539 (84) | 1419 (84) | 120 (82) |
| Maternal substance use (n = 1162), n (%) | 202 (17) | 171 (16) | 31 (27) |
| Maternal viral load, n (%), copies per mL | |||
| ≤400 | 362 (39) | 350 (42) | 12 (13) |
| 401–5000 | 272 (29) | 229 (27) | 43 (46) |
| 5000–50 000 | 235 (25) | 199 (23) | 36 (38) |
| >50 000 | 67 (7) | 64 (8) | 3 (3) |
| Unknown | 904 | 852 | 52 |
IQR indicates interquartile range.
Mean MDI and PDI scores are presented in Tables 2 and 3, respectively, both unadjusted and adjusted for all other covariates in a linear regression model. Overall, unadjusted mean MDI and PDI scores were significantly lower than the US population norms of 100. After controlling for confounders, children who were exposed in utero to any ARV agent showed no decrement in MDI and PDI scores as compared with ARV-unexposed children (adjusted mean MDI: 94.8 vs 92.2 [P = .07]; PDI: 93.9 vs 93.5 [P = .82]). Significantly higher adjusted mean MDI and PDI scores were observed among girls, infants of normal birth weight, and those with more highly educated primary caregivers. In addition, significant variation in both MDI and PDI scores was observed across clinical sites on the basis of site size and of geographic and urban location. Significantly lower adjusted MDI and PDI mean scores were observed in children who were tested with the BSID II as compared with the BSID I. Significant differences among race/ethnicity groups and according to primary language were observed for MDI scores but not for PDI scores. Sensitivity analyses for MDI and PDI scores excluding the effect of birth weight, which may itself be a result of maternal ARV exposure, yielded similar conclusions regarding prenatal exposure (Table 4).
TABLE 2.
Unadjusted and Adjusted MDI Scores by ARV Exposure and Other Characteristics
| Characteristic | n | Unadjusted, Mean ± SD |
Least Squares (Adjusted), Mean |
Pa |
|---|---|---|---|---|
| In utero ARV exposure | .070 | |||
| Not exposed | 146 | 90.6 ± 18.4 | 92.2 | |
| Exposed | 1694 | 87.7 ± 16.9 | 94.8 | |
| BSID test version | <.001 | |||
| BSID I | 449 | 95.4 ± 18.3 | 100.6 | |
| BSID II | 1391 | 85.5 ± 15.9 | 86.4 | |
| Primary caregiver | .580 | |||
| Biological parent | 1758 | 87.9 ± 17.0 | 93.0 | |
| Other adult (foster, adoptive) | 82 | 87.4 ± 17.1 | 93.9 | |
| Primary caregiver education level | <.001 | |||
| Less than high school | 642 | 86.3 ± 17.2 | 90.8 | |
| High school graduate/equivalency | 601 | 87.8 ± 16.6 | 92.0 | |
| Some college/technical school | 401 | 89.2 ± 17.1 | 93.8 | |
| College graduate | 93 | 93.2 ± 18.6 | 98.9 | |
| Other education | 103 | 89.1 ± 15.8 | 92.0 | |
| Primary language | .009 | |||
| English | 1355 | 88.5 ± 17.0 | 94.8 | |
| Other | 485 | 86.4 ± 17.1 | 92.2 | |
| Gender | <.001 | |||
| Male | 919 | 86.0 ± 16.5 | 91.3 | |
| Female | 921 | 89.8 ± 17.4 | 95.6 | |
| Age at time of test, mo | <.001 | |||
| <9 | 209 | 95.5 ± 13.2 | 101.5 | |
| 9–15 | 393 | 94.2 ± 12.9 | 100.0 | |
| 15–21 | 285 | 88.4 ± 15.2 | 92.2 | |
| 21–27 | 804 | 84.0 ± 18.2 | 88.3 | |
| >27 | 149 | 80.7 ± 18.7 | 85.4 | |
| Race/ethnicity | <.001 | |||
| White Non-Hispanic and other race | 242 | 95.3 ± 17.7 | 95.7 | |
| Black non-Hispanic | 1016 | 86.5 ± 16.4 | 91.5 | |
| Hispanic | 582 | 87.4 ± 17.1 | 93.3 | |
| Birth weight | <.001 | |||
| LBW (<2500 g) | 293 | 82.2 ± 16.9 | 90.9 | |
| Normal birth weight (>2500 g) | 1547 | 89.0 ± 16.9 | 96.1 | |
| Birth year | .019 | |||
| 1991–1994 | 294 | 92.7 ± 17.5 | 90.8 | |
| 1994–2000 | 817 | 87.2 ± 17.8 | 94.7 | |
| 2000–2006 | 729 | 86.8 ± 15.7 | 95.0 | |
| Region of United States | <.001 | |||
| Midwest | 165 | 92.0 ± 14.5 | 96.6 | |
| Northeast | 579 | 84.8 ± 17.9 | 89.0 | |
| South (including Puerto Rico) | 851 | 87.8 ± 16.7 | 94.0 | |
| West | 245 | 93.1 ± 16.1 | 94.4 | |
| Type of site | <.001 | |||
| Rural (<1 million population) | 301 | 93.6 ± 15.4 | 97.0 | |
| Urban | 1539 | 86.8 ± 17.1 | 90.0 | |
| Size of site (total accrual) | <.001 | |||
| ≤45 subjects | 1034 | 90.6 ± 16.5 | 96.2 | |
| >45 subjects | 806 | 84.5 ± 17.1 | 90.8 |
From type III F test of effect in multiple linear regression model, adjusting for all other covariates shown above at their average levels.
TABLE 3.
Unadjusted and Adjusted PDI Scores by ARV Exposure and Other Characteristics
| Characteristic | n | Unadjusted, Mean ± SD |
Least Squares (Adjusted), Mean |
Pa |
|---|---|---|---|---|
| In utero ARV exposure | .820 | |||
| Not exposed | 146 | 97.9 ± 20.3 | 93.5 | |
| Exposed | 1694 | 92.9 ± 16.9 | 93.9 | |
| BSID test version | <.001 | |||
| BSID I | 449 | 100.3 ± 19.1 | 98.8 | |
| BSID II | 1391 | 91.0 ± 15.9 | 88.5 | |
| Primary caregiver | .090 | |||
| Biological parent | 1758 | 93.5 ± 17.4 | 95.3 | |
| Other adult (foster, adoptive) | 82 | 89.4 ± 17.2 | 92.1 | |
| Primary caregiver education level | .040 | |||
| Less than high school | 642 | 91.9 ± 18.1 | 91.9 | |
| High school graduate/equivalency | 601 | 94.0 ± 16.4 | 94.0 | |
| Some college/technical school | 401 | 94.7 ± 16.7 | 95.0 | |
| College graduate | 93 | 92.7 ± 16.7 | 94.1 | |
| Other education | 103 | 93.0 ± 18.3 | 93.5 | |
| Primary language | .530 | |||
| English | 1355 | 93.2 ± 16.7 | 93.5 | |
| Other | 485 | 93.4 ± 18.6 | 94.1 | |
| Gender | .025 | |||
| Male | 919 | 92.5 ± 17.3 | 92.8 | |
| Female | 921 | 94.1 ± 17.0 | 94.5 | |
| Age at time of test, mo | <.001 | |||
| <9 | 209 | 93.6 ± 15.5 | 95.1 | |
| 9–15 | 393 | 95.6 ± 14.9 | 97.1 | |
| 15–21 | 285 | 94.3 ± 14.4 | 93.8 | |
| 21–27 | 804 | 92.6 ± 18.4 | 92.8 | |
| >27 | 149 | 88.7 ± 21.8 | 89.7 | |
| Race/ethnicity | .490 | |||
| White Non-Hispanic and other race | 242 | 96.2 ± 16.8 | 94.7 | |
| Black non-Hispanic | 1016 | 92.8 ± 16.4 | 93.4 | |
| Hispanic | 582 | 92.9 ± 18.5 | 93.0 | |
| Birth weight | <.001 | |||
| LBW (<2500 g) | 293 | 88.2 ± 18.4 | 91.2 | |
| Normal birth weight (>2500 g) | 1547 | 94.3 ± 16.8 | 96.1 | |
| Birth year | .450 | |||
| 1991–1994 | 294 | 99.0 ± 20.4 | 92.5 | |
| 1994–2000 | 817 | 93.2 ± 17.0 | 94.4 | |
| 2000–2006 | 729 | 91.1 ± 15.4 | 94.1 | |
| Region of United States | <.001 | |||
| Midwest | 165 | 93.9 ± 14.2 | 94.5 | |
| Northeast | 579 | 89.7 ± 17.3 | 90.2 | |
| South (including Puerto Rico) | 851 | 95.0 ± 17.3 | 96.5 | |
| West | 245 | 95.4 ± 17.0 | 93.7 | |
| Type of site | <.001 | |||
| Rural (<1 million population) | 301 | 96.9 ± 16.7 | 95.7 | |
| Urban | 1539 | 92.6 ± 17.2 | 91.7 | |
| Size of site (total accrual) | <.001 | |||
| ≤45 subjects | 1034 | 94.5 ± 17.2 | 95.8 | |
| >45 subjects | 806 | 91.7 ± 17.0 | 91.6 |
From type III F test of effect in multiple linear regression model, adjusting for all other covariates shown above at their average levels.
TABLE 4.
Adjusted MDI and PDI Scores by ARV Exposure Overall, by Birth Weight, and on the Basis of Separate Models for Each BSID Test Version
| Characteristic | n | MDI |
PDI |
||
|---|---|---|---|---|---|
| Adjusted Meana |
Pb | Adjusted Meana |
Pb | ||
| Overall in utero ARV exposure | .07 | .82 | |||
| Not exposed | 146 | 92.2 | 93.5 | ||
| Exposed | 1694 | 94.8 | 93.9 | ||
| Model excluding adjustment for birth weight | |||||
| Overall in utero ARV exposure | .07 | .82 | |||
| Not exposed | 146 | 93.8 | 95.2 | ||
| Exposed | 1694 | 96.4 | 95.5 | ||
| Model including interaction by birth weight | |||||
| LBW (<2500 g) | .01 | <.01 | |||
| Not exposed | 19 | 82.8 | 79.9 | ||
| Exposed | 274 | 92.8 | 92.3 | ||
| Normal birth weight (≥2500 g) | .73 | .76 | |||
| Not exposed | 127 | 95.9 | 97.8 | ||
| Exposed | 1420 | 97.3 | 96.3 | ||
| Separate models for each BSID test version | |||||
| BSID I | .31 | .36 | |||
| Not exposed | 115 | 96.4 | 102.1 | ||
| Exposed | 334 | 98.4 | 100.0 | ||
| BSID II | .13 | .14 | |||
| Not exposed | 31 | 85.5 | 85.5 | ||
| Exposed | 1360 | 89.3 | 89.7 | ||
Adjusted for age, birth year, sex, race/ethnicity, type of caregiver, caregiver education level, primary language, birth weight (except where indicated), BSID test version (except in separate models by version), region of country, site size, and rural/urban site classification, all at the levels shown in Table 1.
From F test in multiple regression model, adjusting for all of the above covariates.
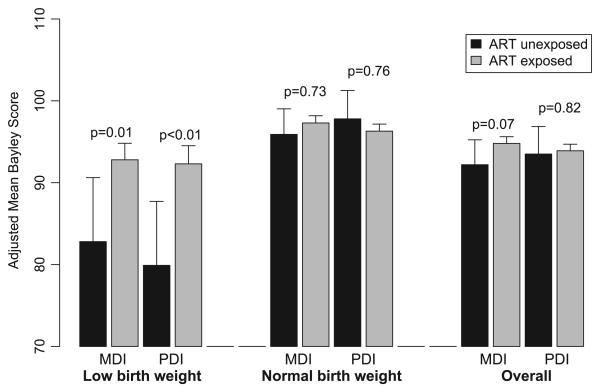
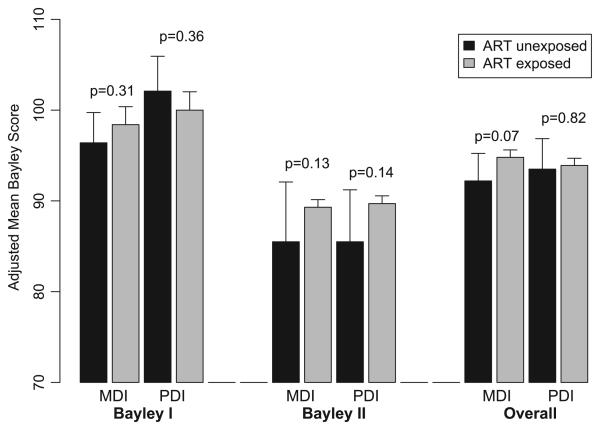
Although no overall effect of maternal ARV exposure was observed, adjusted mean MDI scores were 10 to 12 points higher for ARV-exposed than unexposed among the 293 LBW infants (P = .01; Table 4, Fig 1), whereas no significant difference was observed among infants of normal birth weight. No other significant interactions of covariates with maternal ARV exposure were observed. Separate models fit by BSID test version confirmed results of the overall analysis (Table 4, Fig 2); adjusted MDI and PDI means were within 2 to 4 points for ARV-exposed and unexposed children for each version and were not significantly different.
FIGURE 1.
MDI and PDI scores overall and by birth weight; estimated least squares means for 1840 HIV-exposed uninfected children in PACTG 219/219C at age 2 years, by exposure to ARV treatment (ART). Least squares means are adjusted for exact age at test, birth year, gender, race/ethnicity, type of caregiver, caregiver education level, primary language, region of country, site size, rural/urban site classification, and BSID test version.
FIGURE 2.
MDI and PDI scores overall and by BSID test version (I or II); estimated least squares means for 1840 HIV-exposed uninfected children in PACTG 219/219C at age 2 years, by exposure to ARV treatment (ART). Least squares means are adjusted for exact age at test, birth year, gender, race/ethnicity, type of caregiver, caregiver education level, primary language, birth weight, region of country, site size, and rural/urban site classification.
We also considered several different exposure classifications for maternal ARV, again adjusting for all other covariates in Table 1; however, none of the classifications suggested lower MDI or PDI scores with increasing levels of in utero exposure (data not shown). In particular, we compared infants whose mother received HAART during pregnancy (47%) with those whose mother received a non-HAART regimen (45%) or were unexposed prenatally to ARV (8%) and observed no difference in MDI or PDI scores. The non-HAART regimens (n = 834) included a single NRTI (primarily ZDV; 59%), 2 NRTIs (15%), and other nonHAART combinations (26%). We addressed the effect of regimens, including ZDV plus 3TC, and observed higher adjusted MDI scores in those who were exposed to regimens that contained ZDV + 3TC (57%) than in those who were not exposed (adjusted MDI means: 95.4 vs 91.9; P = .04) but no difference between those who were exposed to other ARV regimens (35%) versus unexposed (mean MDI: 94.2 vs 91.9; P = .16). No difference was observed in adjusted PDI means on the basis of ZDV + 3TC exposure (adjusted means: 93.7 vs 94.0 vs 93.6 for ZDV + 3TC-containing, other ARV, and unexposed, respectively).
We found a significant trend of higher mental scores with increasing duration of maternal ARV exposure, with adjusted mean MDI scores of 92.4, 94.5, 95.3, and 95.9 for infants who were exposed 0 weeks (9%), 1 to 13 weeks (23%), 13 to 25 weeks (36%), and ≥26 weeks (32%), respectively (P = .02); however, mean scores were only ~3 points higher for those who were exposed for ≥26 weeks as compared with unexposed children. Trimester of in utero exposure could be adequately ascertained for 1614 (88%) infants; mean MDI scores for children whose mother received a ZDV + 3TC- containing regimen were marginally higher for second-trimester exposure and significantly higher (~3 points) for third-trimester exposure, as compared with those who were not exposed during that respective trimester.
Maternal substance use during pregnancy was reported for 17% of mothers with available information but was not associated with MDI or PDI scores (Table 5). Among the subset of 936 (51%) infants with maternal viral load information available from other PACTG perinatal treatment trials, there were slightly lower (but not statistically significant) adjusted MDI scores with increasing viral load, from 93.9 for those with <400 copies per mL to 89.1 for those with >50 000 copies per mL (trend test P = .09), and a parallel significant decline in adjusted PDI scores (trend test P = .03). In this subset, there was no overall effect of maternal ARV exposure after adjustment for maternal viral load, but the significant increase of ~10 points in MDI scores among LBW infants who were exposed to ARV as compared with unexposed LBW infants was confirmed.
TABLE 5.
Adjusted MDI and PDI Scores by ARV Exposure Within Subsets With Maternal Viral Load and Maternal Substance Use Information
| Characteristic | n | MDI |
PDI |
||
|---|---|---|---|---|---|
| Adjusted Meana |
Pb | Adjusted Meana |
Pb | ||
| Among those with maternal substance use data (n = 1162) | |||||
| In utero ARV exposure | .46 | .32 | |||
| Not exposed | 116 | 92.6 | 95.3 | ||
| Exposed | 1046 | 93.9 | 93.3 | ||
| Maternal substance use | .69 | .94 | |||
| Yes | 202 | 93.0 | 94.3 | ||
| No | 960 | 93.5 | 94.4 | ||
| Among those with maternal viral load data (n = 936) | |||||
| In utero ARV exposure | .83 | .13 | |||
| Not exposed | 94 | 91.8 | 92.6 | ||
| Exposed | 842 | 92.2 | 89.0 | ||
| Maternal viral load, copies per mL | .12 | .06 | |||
| ≤400 | 362 | 93.9 | 93.5 | ||
| 401–5000 | 272 | 92.0 | 91.0 | ||
| 5000–50 000 | 235 | 93.0 | 91.6 | ||
| >50 000 | 67 | 89.1 | 87.1 | ||
Adjusted for age, birth year, gender, race/ethnicity, type of caregiver, caregiver education level, primary language, birth weight, BSID test version, region of country, site size, and rural/urban site classification, all at the levels shown in Table 1.
From F test in multiple regression model, adjusting for all of the above covariates; for maternal viral load, from trend test of decreasing scores with increasing viral load.
Last, logistic regression models supported a lack of association between in utero ARV exposure and higher percentages of children with mental or motor impairment (defined by BSID scores <70). The prevalence of mental impairment was 13% within each exposure group and for motor functioning was 10% within each group. Multiple logistic regression models that adjusted for the covariates described previously suggested a decreased odds of mental impairment for ARV-exposed versus unexposed infants (odds ratio: 0.50; P = .05) and no difference in motor impairment rates (odds ratio: 0.85; P = .65).
DISCUSSION
Our study of >1800 children who were born to HIV-infected mothers found no decrement in neurodevelopmental functioning at 2 years of age associated with in utero exposure to ARV agents. After adjustment for important covariates that are associated with mental and motor functioning, similar or slightly higher mean BSID scores were observed among those with prenatal ARV exposure as compared with those without. These relationships were maintained even in separate models fit for each BSID test version, which is notable given the shift in mean scores between these 2 versions.38 These results provide additional support to previous studies that found no association between early neurodevelopmental functioning and prenatal ARV exposure and also provide reassurance regarding the safety of in utero ARV exposure.
Alimenti et al33 observed significantly lower mean MDI scores for 39 HAART-exposed children as compared with 24 unexposed children and consistently lower scores across a number of other developmental assessments; however, their study had unexpected imbalances between their ARV-exposed group and their “control” group in maternal illicit drug use and other important confounders. A previous analysis of >800 children with the BSID II test by Lindsey et al32 found no difference in mental and motor functioning between infants who were younger than 1 year who did or did not have maternal ARV exposure; however, this was not the primary focus of their evaluation and was not adjusted for any potential confounders.
High rates of illicit drug use during pregnancy have been reported for HIV-infected women,40-42 which could pose risks for infant neurodevelopment; however, we did not observe a relationship between self-reported maternal illicit drug use and cognitive or motor functioning, which is consistent with findings from a number of other large studies.31,32,40 The WITS found a delay in mental and motor functioning at 4 months of age but not at 24 months among HIV-exposed uninfected children with maternal hard drug exposure.40 Similarly, other evaluations of the WITS data have found no effect of prenatal illicit drug exposure on mental or motor scores through the first 30 months of life31 or on behavioral outcomes.42 Previous evaluations of the PACTG 219/219C cohort also identified no effect of maternal injection drug use on BSID scores.32 However, it is possible that self-reported maternal substance use in our study is under-reported because of social desirability, fear of legal consequences, and concerns regarding child custody issues; such underreporting may attenuate associations.41
Although we observed slightly higher BSID scores for ARV-exposed children after adjustment for demographic and socioeconomic measures, these differences were small and may reflect incomplete control for other potential confounders, such as household income levels and access to prenatal care. We found significantly higher mental functioning among LBW infants who were prenatally exposed to ARV, which suggests the possibility of greater access to prenatal care as compared with the ARV-unexposed LBW infants. The additional advantages of both prenatal and postnatal care may be most dramatic in situations in which the infant's health is at risk, such as for LBW infants. Such care may include skilled neonatal attention along with health and nutritional counseling for both infants and their mothers, which in turn may create a home environment that is conducive to appropriate neurodevelopment; however, greater access to prenatal care would not be expected for those who originally were randomly assigned to placebo in AIDS Clinical Trials Group 076, which comprised 68% of the unexposed individuals in our analysis. We also observed a trend suggestive of lower neurodevelopmental functioning with higher maternal viral load, suggesting the possibility of a cytokine response associated with incompletely controlled viral load during pregnancy.43
The major strengths of our study are its large size, its prospective nature, and its ability to control for many potential confounders. In addition, the inclusion of a subgroup of ARV-unexposed children is a key strength that is unlikely to be repeated in future studies, at least in high-resource settings; however, the observational nature of the study is a limitation in that there may be unmeasured confounders, which could lead to biases in estimated effects.44 Assessment of neurodevelopmental functioning among ARV-exposed uninfected children as they age into school years is warranted, along with continued monitoring to address the safety of new ARV agents and combination regimens received by HIV-infected pregnant women.
WHAT'S KNOWN ON THIS SUBJECT
Two previous studies evaluated the effects of prenatal ARV exposure on neurodevelopmental functioning in HIV-exposed uninfected infants and found no association; however, these studies were limited by sample size or lack of adjustment for potential confounders.
WHAT THIS STUDY ADDS
We evaluated the association of prenatal ARV exposure with neurodevelopmental functioning in >1800 HIV-exposed uninfected infants, adjusting for many potential confounders. We found no decrement in functioning of ARV-exposed 2-year-olds, providing reassurance regarding the safety of in utero ARV exposure.
ACKNOWLEDGMENTS
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts N01-3-3345 and HHSN267200800001C). Support for the Statistical and Data Analysis Center at Harvard School of Public Health was provided under the National Institute of Allergy and Infectious Diseases cooperative agreement 5 U01 AI41110 with the PACTG) and 1 U01 AI068616 with IMPAACT.
The following institutions and clinical site investigators participated in PACTG Protocol 219C: Baylor Texas Children's Hospital: F. Minglana, M.E. Paul, C.D. Jackson; University of Florida, Jacksonville: M.H. Rathore, A. Khayat, K. Champion, S. Cusic; Chicago Children's Memorial Hospital: R. Yogev, E. Chadwick; University of Puerto Rico, University Children's Hospital AIDS Program: I. Febo-Rodriguez, S. Nieves; Bronx Lebanon Hospital Center; M. Purswani, S. Baksi, E. Stuard, M. Dummit; San Juan Hospital: M. Acevedo, M. Gonzalez, L. Fabregas, M.E. Texidor; University of Miami: G.B. Scott, C.D. Mitchell, L. Taybo, S. Willumsen; University of Medicine and Dentistry of New Jersey: L. Bettica, J. Amour, B. Dashefsky, J. Oleske; Charity Hospital of New Orleans and Earl K. Long Early Intervention Clinic: M. Silio, T. Alchediak, C. Boe, M. Cowie; UCSD Mother, Child and Adolescent HIV Program: S.A. Spector, R. Viani, M. Caffery, L. Proctor; Howard University: S. Rana, D. Darbari, J.C. Roa, P.H. Yu; Jacobi Medical Center: M. Donovan, R. Serrano, M. Burey, R. Auguste; St Christopher's Hospital for Children, Philadelphia: J. Chen, J. Foster; Bay-state Medical Center Children's Hospital: B.W. Stechenberg, D.J. Fisher, A.M. Johnston, M. Toye; Los Angeles County Medical Center/USC: J. Homans, M. Neely, L.S. Spencer, A. Kovacs; Children's Hospital Boston: S. Burchett, N. Karthas; Children's Hospital of Michigan: E. Moore, C. Cromer; St Jude Children's Research Hospital, Memphis: P.M. Flynn, N. Patel, M. Donohoe, S. Jones; New York University School of Medicine/Bellevue Hospital: W. Borkowsky, S. Chandwani, N. Deygoo, S. Akleh; Children's Hospital at Down-state: E. Handelsman, H.J. Moallem, D.M. Swindell, J.M. Kaye; Columbia Presbyterian Medical Center and Cornell University New York Presbyterian Hospital: A. Higgins, M. Foca, P. LaRussa, A. Gershon; Children's Hospital of Philadelphia: R.M. Rutstein, C.A. Vincent, S.D. Douglas, G.A. Koutsoubis; Children's Hospital of Oakland: A. Petru, T. Courville; UCSF, Moffitt Hospital: D. Wara, D. Trevithick; Children's Hospital, University of Colorado, Denver: E. McFarland, C. Salbenblatt; Johns Hopkins University Pediatrics: N. Hutton, B. Griffith, M. Joyner, C. Kiefner; Children's Hospital and Regional Medical Center, Washington: M. Acker, R. Croteau, C. McLellan, K. Mohan; Metropolitan Hospital Center: M. Bamji, I. Pathak, S. Manwani, E. Patel; Children's National Medical Center: H. Spiegel, V. Amos; University of Massachusetts Medical School: K. Luzuriaga; University of Alabama at Birmingham: R. Pass, M. Crain; University of Maryland Medical Center: J. Farley, K. Klipner; Schneider Children's Hospital: V.R. Bonagura, S.J. Schuval, C. Colter, L. Campbell; Boston Medical Center: S.I. Pelton, A.M. Reagan; University of Illinois: K.C. Rich, K. Hayani, M. Bicchinella; SUNY Stony Brook: S. Nachman, D. Ferraro, S. Madjar; North Broward Hospital District: A. Puga; Duke University: F. Wiley, K. Whitfield, O. Johnson, R. Dizney; Harlem Hospital: S. Champion, M. Frere, M. DiGrado, E.J. Abrams; Cook County Hospital: J. Martinez; University of South Alabama: M. Mancao; Connecticut Children's Medical Center: J. Salazar, G. Karas; University of North Carolina at Chapel Hill: T. Belho, B. Pitkin, J. Eddleman; Ruiz Arnau University Hospital: W. Figueroa, E. Reyes; SUNY Upstate Medical University: L.B. Weiner, K.A. Contello, W.A. Holz, M.J. Famiglietti; Children's Medical Center of Dallas; University of Florida at Gainesville: R. Lawrence, J. Lew, C. Delany, C. Duff; Children's Hospital at Albany Medical Center: A.D. Fernandez, P.A. Hughes, N. Wade, M.E. Adams; Lincoln Medical and Mental Health Center; Phoenix Children's Hospital: J.P. Piatt, J. Foti, L. Clarke-Steffen; Public Health Unit of Palm Beach County: J. Sleasman, C. Delaney; Medical College of Georgia: C.S. Mani; Yale University School of Medicine: W.A. Andiman, S. Romano, L. Hurst, J. de Jesus; Vanderbilt University Medical Center: G. Wilson; University of Rochester Medical Center: G.A. Weinberg, F. Gigliotti, B. Murante, S. Laverty; St Josephs Hospital and Medical Center, New Jersey: N. Hutchcon, A. Townley; Emory University Hospital: S. Nesheim, R. Dennis; University of South Florida: P. Emmanuel, J. Lujan-Zilberman, C. Graisberry, S. Moore; Children's Hospital of the King's Daughters: R.G. Fisher, K.M. Cunnion, T.T. Rubio, D. Sandifer; Medical University of South Carolina: G.M. Johnson; University of Mississippi Medical Center: H. Gay, S. Sadler; Harbor-UCLA Medical Center: M. Keller, J. Hayes, A. Gagajena, C. Mink; Mount Sinai Medical Center: D. Johnson; Children's Hospital of Los Angeles: J. Church, T. Dunaway, C. Salata; Long Beach Memorial: A. Deveikis, L. Melton; Robert Wood Johnson Medical School: S. Gaur, P. Whitley-Williams, A. Malhotra, L. Cerracchio; Sinai Children's Hospital: M. Dolan, J. D'Agostino, R. Posada; Medical Center, Pediatric Columbus, Georgia: C. Mani, S. Cobb; Medical College of Virginia: S.R. Lavoie, T.Y. Smith; Cooper Hospital-University Medical Center: A. Feingold, S. Burrows-Clark; University of Cincinnati: J. Mrus, R. Beiting; Columbus Children's Hospital: M. Brady, J. Hunkler, K. Koranyi; Sacred Heart Children's CMS of Florida: W. Albritton; St Luke's/Roosevelt Hospital Center: R. Warford, S. Arpadi; Incarnation Children's Center, New York: A. Gershon, P. Miller; Montefiore Medical-AECOM: A. Rubinstein, G. Krienik; Children's Hospital of Los Angeles: A. Kovacs, E. Operskalski; San Francisco General Hospital: D. Wara, A. Kamrin, S. Farrales; Cornell University New York Presbyterian: R. Johan-Liang, K. O'Keefe; St Louis Children's Hospital: K.A. McGann, L. Pickering, G.A. Storch; North Shore University Hospital: S. Pahwa, L. Rodriquez; Oregon Health and Science University: P. Lewis, R. Croteau.
We thank the children and families for participation in PACTG 219C and the individuals and institutions involved in the conduct of 219C as well as the leadership and participants of the P219/219C protocol team (James M. Oleske, MD, MPH, Founding Chair, and Russell Van Dyke, MD, Chair; Mark Abzug, MD, and John Farley, MD; Vice-Chairs, Mary Glen Fowler, MD, MPH, Michael Brady, MD, and Wayne Dankner, MD; Past Vice-Chairs, Protocol Team Members (versions 1 and 2 of PACTG-219): Mary Culnane, MS, CRNP, Elizabeth Hawkins, Lynne Mofenson, MD, Yvonne J. Bryson, MD, Edward M. Connor, MD, Lawrence D'Angelo, MD, MPH, Mark Mintz, MD, Karen J. O'Donnell, PhD, Margaret Ox-toby, MD, Andrea Rubin Hale, RN, MPH, Richard D. Gelber, PhD, Steven Gort-maker, PhD, William Lenderking, PhD, Lynn Marrow, Christina Joy, RN, MSM, Colleen Clark, MPH, Bethann Cunningham, MS, Rhoda Sperling, MD, Gwen-dolyn B. Scott, MD, Courtney Fletcher, PharmD, Blake Caldwell, MD, Dianne Donovan. Protocol Team Members (versions 3 and 4 of PACTG-219C): Elizabeth Smith, MD, Anne Fresia, Gregory Ciupak, Carol Elgie, Michelle Eagle, PA, Dorothy R. Smith, MS, CPNP, Paul Palumbo, MD, John Sleasman, MD, James Connor, MD, Michael Hughes, PhD, Rebecca Oyomopita, MSc, George Johnson, MD, Andrew Wiznia, MD, Nancy Hutton, MD, Andrea Kovacs, MD, Mary Sawyer, MD, Martin Anderson, MD, Audrey Rogers, PhD, MPH, William Borkowsky, MD, Jane Lindsey, ScD, Jack Moye, MD, Myron Levin, MD, Marilyn Crain, MD, MPH, Paul Britto, MS, Ruth Toumala, MD, Joseph Cervia, MD, Eileen Monagham, Kenneth Dominguez, MD, Melody Higgins, RN, MS, George Seage, DSc, MPH, Denise Gaughan, MPH, Phil Gona, PhD, William Shearer, MD, PhD, Lois Howland, DPH, MS, RN, Deborah Storm, PhD, RN, Kathleen Malee, PhD, Wendy Mitchell, MD, Carol Gore, Eve Powell, Michelle McConnell, MD, Newana Beatty, Susan Brogly, PhD, Jennifer Bryant, CRA, Miriam Chernoff, PhD, Barbara Heckman, BS, Dawn English, Edward Handelsman, MD, Patrick Jean-Philippe, MD, Kathleen Kaiser, Joyce Kraimer, MS, Linda Millar, Shirley Traite, MSW, Paige Williams, PhD, Elizabeth Woods, MD, MPH, Carol Worrell, MD). We are grateful for the contributions of Joyce Kraimer, Barbara Heckman, Shirley Traite, and Nathan Tryon. We also thank the individual staff members and sites who have participated in the conduct of this study, as provided in the previous paragraph.
ABBREVIATIONS
- ARV
antiretroviral
- MTCT
mother-to-child transmission
- ZDV
zidovudine
- HAART
highly active antiretroviral therapy
- NRTI
nucleoside reverse transcriptase inhibitor
- 3TC
lamivudine
- WITS
Women and Infants Transmission Study
- PACTG
Pediatric AIDS Clinical Trials Group
- BSID
Bayley Scales of Infant Development
- MDI
Mental Developmental Index
- PDI
Psychomotor Developmental Index
- LBW
low birth weight
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1 infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29(5):484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ioannidis JP, Abrams EJ, Ammann A, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads < 1000 copies/ml. J Infect Dis. 2001;183(4):539–545. doi: 10.1086/318530. [DOI] [PubMed] [Google Scholar]
- 4.Suksomboon N, Poolsup N, Ket-aim S. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32(3):293–311. doi: 10.1111/j.1365-2710.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 5.Perinatal HIV Guidelines Working Group Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. 2009 April 29;:1–90. Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. Accessed November 23, 2009.
- 6.Ayers KM, Clive D, Tucker WE, Jr, Haijian G, de Miranda P. Nonclinical toxicology studies with zidovudine: genetic toxicity and carcinogenicity bioassays in mice and rates. Fundam Appl Toxicol. 1996;32(2):148–158. doi: 10.1006/faat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 7.Olivero OA, Anderson LM, Diwan BA, et al. Transplacental effects of 3′-azido-2′,3′-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J Natl Cancer Inst. 1997;89(21):1602–1608. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 8.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol. 2004;199(2):151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Culnane M, Fowler MG, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA. 1999;281(2):151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 10.Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern? Drug Saf. 2007;30(30):203–213. doi: 10.2165/00002018-200730030-00004. [DOI] [PubMed] [Google Scholar]
- 11.European Collaborative Study Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32(4):380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Sperling RS, Shapiro DE, McSherry GD, et al. Safety of the maternal-infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 study. AIDS. 1998;12(14):1805–1813. doi: 10.1097/00002030-199814000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Hanson IC, Antonelli A, Sperling RS, et al. Lack of tumors in infants with perinatal HIV-1 exposure and fetal/neonatal exposure to zidovudine. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(5):463–467. doi: 10.1097/00042560-199904150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Brogly S, Williams P, Seage GR, et al. In utero nucleoside reverse transcriptase inhibitor exposure and cancer in HIV-uninfected children: an update from the pediatric AIDS clinical trials group 219 and 219C cohorts. J Acquir Immune Defic Syndr. 2006;41(4):535–536. doi: 10.1097/01.qai.0000194735.66322.d9. [DOI] [PubMed] [Google Scholar]
- 15.The European Collaborative Study Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111(1) doi: 10.1542/peds.111.1.e52. Available at: www.pediatrics.org/cgi/content/full/111/1/e52. [DOI] [PubMed] [Google Scholar]
- 16.Moye J, Rich KC, Kalish LA. Natural history of somatic growth in infants born to women infected by human immunodeficiency virus. J Pediatr. 1996;128(1):58–69. doi: 10.1016/s0022-3476(96)70428-6. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco SE, McIntosh K, Lu M, Mofenson LM, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIVuninfected children: an analysis of the Women and Infants Transmission Study. J Infect Dis. 2006;194(8):1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 18.Le Chenadec J, Mayaux M-J, Giuhenneuc-Jouyaux C, Blanche S, Enquete Perinatale Francaise Study Group Perinatal antiretroviral treatment and hematopoiesis in HIVuninfected infants. AIDS. 2003;17(14):2053–2061. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bunders MJ, Bekker V, Scherpbier HJ, et al. Haematological parameters of HIV-1-uninfected infants born to HIV-1-infected mothers. Acta Paediatr. 2005;94(11):1571–1577. doi: 10.1080/08035250510042951. [DOI] [PubMed] [Google Scholar]
- 20.European Collaborative Study Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1 infected mothers. AIDS. 2004;18(15):2009–2017. doi: 10.1097/00002030-200410210-00005. [DOI] [PubMed] [Google Scholar]
- 21.Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25(3):261–268. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
- 22.Lindegren ML, Rhodes PH, Gordon L, et al. Drug safety during pregnancy and in infants: lack of mortality related to mitochondrial dysfunction among perinatally HIV-exposed children in pediatric HIV surveillance. Ann N Y Acad Sci. 2000;918:222–235. [PubMed] [Google Scholar]
- 23.Mofenson LM, Muderi P. Safety of antiretroviral prophylaxis of perinatal transmission for HIV-infected pregnant women and their infants. J Acquir Immune Defic Syndr. 2002;30(2):200–215. doi: 10.1097/00042560-200206010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Bulterys M, Nesheim S, Abrams EJ, et al. Lack of evidence of mitochondrial dysfunction in the offspring of HIV-infected women: retrospective review of perinatal exposure to antiretroviral drugs in the Perinatal AIDS Collaborative Transmission Study. Ann N Y Acad Sci. 2000;918:212–221. doi: 10.1111/j.1749-6632.2000.tb05491.x. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez K, Bertoli J, Fowler M, et al. Lack of definitive severe mitochondrial signs and symptoms among deceased HIV-uninfected and HIV-indeterminate children < 5 years of age. Ann N Y Acad Sci. 2000;918:236–246. doi: 10.1111/j.1749-6632.2000.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 26.Lipshultz SE, Easley KA, Orav EJ, et al. Absence of cardiac toxicity of zidovudine in infants. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 2000;343(11):759–766. doi: 10.1056/NEJM200009143431102. [DOI] [PubMed] [Google Scholar]
- 27.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354(9184):1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 28.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17(12):1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Brogly SB, Ylitalo N, Mofenson LM, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21(8):929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 30.Poirier MC, Divi RL, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIVuninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33(2):175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 31.Chase C, Ware J, Hittelman J, Blasini I, et al. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Women and Infants Transmission Study Group. Pediatrics. 2000;106(2) doi: 10.1542/peds.106.2.e25. Available at: www.pediatrics.org/cgi/content/full/106/2/e25. [DOI] [PubMed] [Google Scholar]
- 32.Lindsey JC, Malee KM, Brouwers P, Hughes MD, PACTG 219C Study Team Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119(3) doi: 10.1542/peds.2006-1145. Available at: www.pediatrics.org/cgi/content/full/119/3/e681. [DOI] [PubMed] [Google Scholar]
- 33.Alimenti A, Forbes JC, Oberlander TF, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics. 2006;118(4) doi: 10.1542/peds.2006-0525. Available at: www.pediatrics.org/cgi/content/full/118/4/e1139. [DOI] [PubMed] [Google Scholar]
- 34.Gortmaker S, Hughes M, Cervia J, et al. Effect of combination therapy including pro-tease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345(21):1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 35.Gona P, van Dyke R, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 36.Bayley N. Manual for the Bayley Scales of Infant Development. Psychological Corporation; San Antonio, TX: 1969. [Google Scholar]
- 37.Bayley N. Manual for the Bayley Scales of Infant Development. 2nd ed. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 38.Schuler ME, Nair P, Harrington D. Developmental outcome of drug-exposed children through 30 months: a comparison of Bayley and Bayley-II. Psychol Assess. 2003;15(3):435–438. doi: 10.1037/1040-3590.15.3.435. [DOI] [PubMed] [Google Scholar]
- 39.Brogly S, Williams P, Seage GR, III, et al. Anti-retroviral treatment in pediatric HIV in the United States: from clinical trials to clinical practice. JAMA. 2005;293(18):2213–2220. doi: 10.1001/jama.293.18.2213. [DOI] [PubMed] [Google Scholar]
- 40.Macmillan C, Magder LS, Brouwers P, et al. Head growth and neurodevelopment of infants born to HIV-1-infected drug-using women. Neurology. 2001;57(8):1402–1411. doi: 10.1212/wnl.57.8.1402. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez EM, Mendez H, Rich K, et al. Maternal drug use in perinatal HIV studies: the Women and Infants Transmission Study. Ann N Y Acad Sci. 1993;693:245–248. doi: 10.1111/j.1749-6632.1993.tb26272.x. [DOI] [PubMed] [Google Scholar]
- 42.Mellins CA, Smith R, O'Driscoll P, et al. High rates of behavioral problems in perinatally HIV-infected children are not linked to HIV disease. Pediatrics. 2003;111(2):384–393. doi: 10.1542/peds.111.2.384. [DOI] [PubMed] [Google Scholar]
- 43.Mercier F, Boulassel MR, Yassine-Diab B, et al. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Ralpha on central and effector memory CD4+ and CD8+ T cell subsets. Clin Exp Immunol. 2008;152(1):72–80. doi: 10.1111/j.1365-2249.2008.03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes MD, Williams PL. Challenges in using observational studies to evaluate adverse effects of treatment. N Engl J Med. 2007;356(17):1705–1707. doi: 10.1056/NEJMp078038. [DOI] [PubMed] [Google Scholar]