Figure 2.
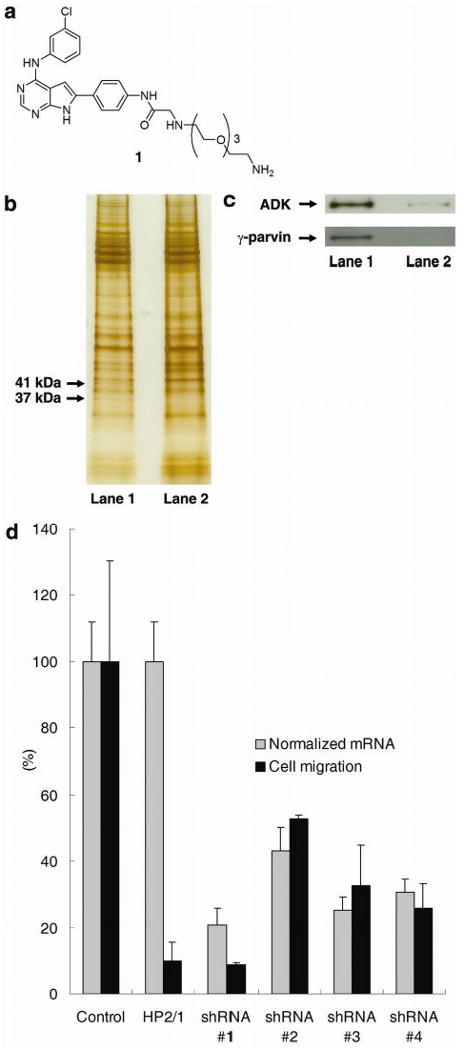
(a) Structure of ligand 1 for affinity matrix. (b) Silver-stained SDS-PAGE gel showing γ-parvin and ADK proteins pulled down by affinity matrix. Lane 1: cell extracts were treated with DMSO (1%); Lane 2: cell extracts were treated with JK273 (100 μM). (c) Confirmation of γ-parvin and ADK identity by Western blot analysis. Lane 1: cell extracts were treated with DMSO (1%); Lane 2: cell extracts were treated with JK273 (100 μM). d) Boyden chamber assay with Jurkat cells and quantitative RT-PCR analysis of γ-parvin expression after infection with lentiviruses producing shRNAs targeting γ-parvin for 84 h. An anti-α4 integrin antibody (clone HP2/1) was used as a positive control for inhibition of cell migration in the Boyden chamber assay. GAPDH was used as an internal control for quantitative RT-PCR analysis. Data are mean ± S.D.
