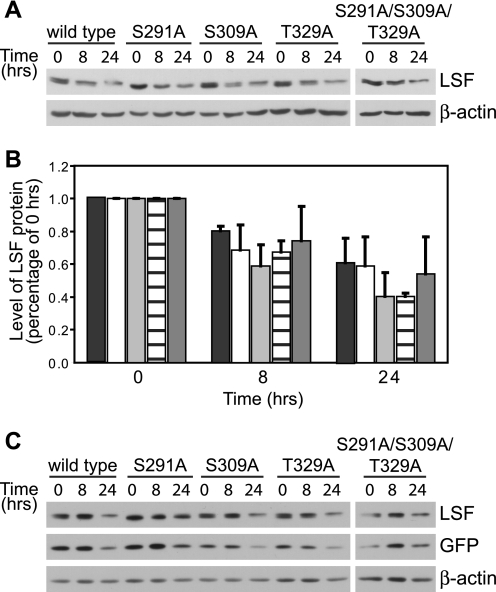
FIGURE 5.
The ability of LSF to bind Pin1 does not alter its protein stability. A, wild-type or each indicated LSF mutant was expressed in exponentially growing NIH 3T3 cells, and cell extracts were harvested before (0 h) or after (8 and 24 h) cycloheximide addition. A representative analysis of the cell extracts from one experiment is shown, immunoblotted using anti-LSF-314 antibody (upper panel). Subsequent immunoblotting using β-actin antibodies validated equal sample loading (lower panel). B, immunoblots as in panel A were quantified for two independent experiments and normalized to levels of each LSF protein at the 0 h time point. The indicated bars represent quantification of data for wild-type LSF (black), LSF S291A (white), LSF S309A (light gray), T329A LSF (horizontal lines), and S291A/S309A/T329A LSF (dark gray). Error bars represent S.E. C, NIH 3T3 cells were transfected as above with plasmids co-expressing GFP and wild-type or mutant LSF and treated with cycloheximide for 0, 8, and 24 h. Cell extracts (20 μg of each) were immunoblotted for LSF (anti-LSF-314 antibody), GFP, and β-actin. A representative experiment is shown; relative levels of LSF compared with GFP levels, normalizing for wild-type LSF at 0 h, ranged from 0.93 to 1.46. This indicates similar decay rates of all LSF proteins and GFP. Furthermore, combining quantification of two independent experiments, there were no statistical differences in decay rates of wild-type versus mutant LSF proteins, using GFP to normalize the data.