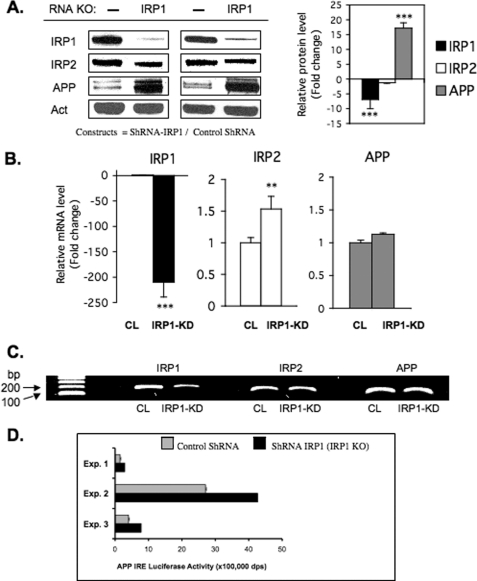
FIGURE 4.
Stable knockdown of IRP1 increases the expression of APP in shRNA transfected H4 neural cells. A, total protein extracts were prepared from SH-SY5Y cells stably expressing shRNAs against human IRP1. A non-targeting siRNA sequence was transfected as a control. IRP1 and APP protein levels were determined by Western blot in control cells and IRP1 knockdown cells, and β-actin (Act) was used as a control (n = 4). The relative change of IRP1, IRP2, and APP protein levels was quantitated in two of the clones (left panel) and presented as -fold change (histogram; right panel). B, real-time qPCR (n = 6) was carried out on the ABI Prism 7000 sequence detection system (Applied Biosystems). Total RNA was isolated using TRIzol reagent (Sigma) according to the manufacturer's instructions. cDNA was synthesized with SuperScript III first-strand qPCR supermix (Invitrogen) according to the manufacturer's instructions. The primers (IRP1, IRP2, β-actin, TfR1) were designed as in Ref. 31 and ordered from Invitrogen. The APP primer set was purchased from Qiagen and has been benchmarked on several reports for accurate measurement of APP mRNA levels (62). CL, cell lysate. KD, knockdown. C, representative 2% agarose gel employed during qRT-PCR and analysis to generate the data shown in panel B. D, three separate reporter assays (Exp. 1–3) registering APP 5′-UTR-luciferase activity in IRP1 shRNA knockdown cells relative to normal IRP1 expression (empty shRNA control).