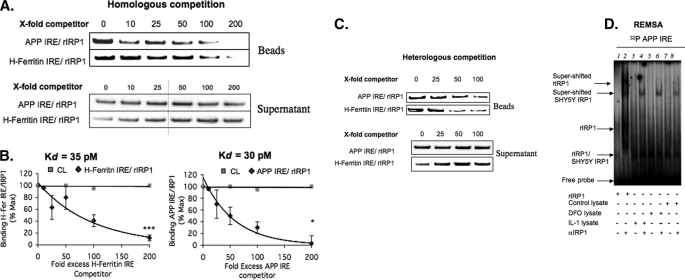
FIGURE 5.
rhIRP1 binds to APP IRE and H-ferritin IRE probes with similar binding affinity in vitro. Competition assays were employed (n = 4) with rhIRP1 and the use of incrementally increasing concentrations of APP IRE and H-ferritin-IRE RNAs in the presence of biotinylated RNA probes (“Experimental Procedures”). A, homologous competition (n = 3). rhIRP1 was incubated with either 25 nm biotinylated APP IRE or H-ferritin IRE in the presence of a 0-, 10-, 25-, 50-, 100-, or 200-fold (25, 250, 625, 1250, 2500, or 5000 nm) excess of unlabeled APP IREs or H-ferritin IRE. B, Scatchard plots from the quantitated data of panel A were used to calculate the dissociation constants (Kd) of rhIRP1 binding to H-ferritin IRE (left side) or APP IRE (right side). C, heterologous competition (n = 3). 25 nm biotinylated APP IRE was competed with 0-, 25-, 50-, or 100-fold unlabeled H-ferritin IRE, and likewise, 25 nm biotinylated H-ferritin IRE was competed with 0-, 25-, 50-, or 100-fold unlabeled APP IRE. D, RNA gel-shift analysis was performed with the 37-nt radiolabeled RNA probe encoding APP IRE (37 nt) sequences as used in the biotin pulldown assays (previously reported by Rogers et al. (1)), including a supershift assay demonstrating that the APP IRE probes employed for biotin pulldown assays detected rhIRP1- and SH-SY5Y-specific IRP1 binding with the same specificity. Lane 1, rhIRP1; lane 2, rhIRP + IRP-specific antibody; lane 3 SH-SY5Y/DFO, 100 μm; lane 4, SH-SY5Y/DFO 100 μm + IRP Ab; lane 5, SH-SY5Y/IL 1β; lane 6, SH-SY5Y/IL-1β + IRP Ab; lane 7 SH-SY5Y/Control; Lane 8, SH-SY5YControl + IRP1 Ab.