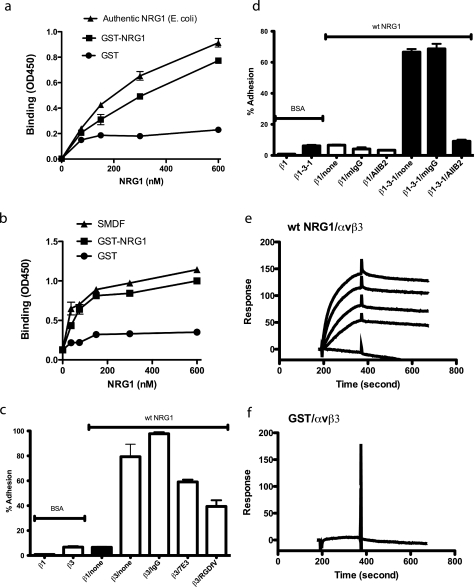
FIGURE 1.
Direct binding of the EGF-like domain of NRG1 to integrin αvβ3. a and b, the EGF-like domain of NRG1 bound to recombinant soluble αvβ3 in a dose-dependent manner in ELISA-type assays. The EGF-like domain peptide of NRG1 (NRG1α, synthesized in E. coli, R&D systems) (a), SMDF, an isoform of NRG1 (synthesized in eukaryotic cells, R&D systems) (b), GST fusion protein of NRG1, or control GST was immobilized to wells of 96-well microtiter plates. The concentrations of the coating solution are shown. Soluble recombinant integrin αvβ3 (5 μg/ml) was added to the wells in the presence of 1 mm Mn2+ and incubated for 2 h at room temperature. After washing the wells, bound αvβ3 was determined by using anti-β3 antibody and HRP-conjugated anti-mouse IgG. The data are shown as the means ± S.E. of triplicate experiments. c, specific adhesion of CHO cells that express human β3 (β3-CHO) to NRG1 is shown. Wells of 96-wellmicrotiter plate were coated with NRG1 (580 nm) or BSA, and the remaining protein-binding sites were blocked with BSA. Wells were incubated with β3-CHO cells or β1-CHO cells for 1 h at 37 °C in Tyrode's-HEPES buffer with 1 mm MgCl2. Bound cells were quantified. mAb 7E3 (to human β3, 10 μg/ml) and cyclic RGDfV (specific antagonist to αvβ3, 10 μm) blocked the adhesion of β3-CHO cells to WT NRG1. IgG represents purified mouse IgG used as a control. The data are shown as the means ± S.E. of triplicate experiments. d, adhesion of CHO cells that express human β1 (β1-CHO) or β1-3-1 (β1-3-1-CHO) to NRG1 is shown. The β1-3-1 mutation changes the specificity of β1 integrins to that of β3 integrins. Cell adhesion was performed as described above. β1-3-1-CHO cells adhered to WT NRG1, and this binding was blocked by anti-human β1 antibody AIIB2 (10 μg/ml) but not by purified mouse IgG (mIgG). BSA as a control was coated instead of NRG1. The data are shown as the means ± S.E. of triplicate experiments. The results suggest that NRG1 specifically binds to β1-3-1 (as αvβ1-3-1) but not to WT β1 (as αvβ1). e and f, surface plasmon resonance studies of NRG1-αvβ3 interaction are shown. Soluble integrin αvβ3 was immobilized to a sensor chip, and the binding of WT NRG1 and control GST (concentrations at 1000, 500, 250, 125, and 0 nm) was analyzed in the presence of 1 mm MnCl2. KD was calculated as 1.36 × 10−7 m for WT NRG-1. Control GST did not show significant binding.