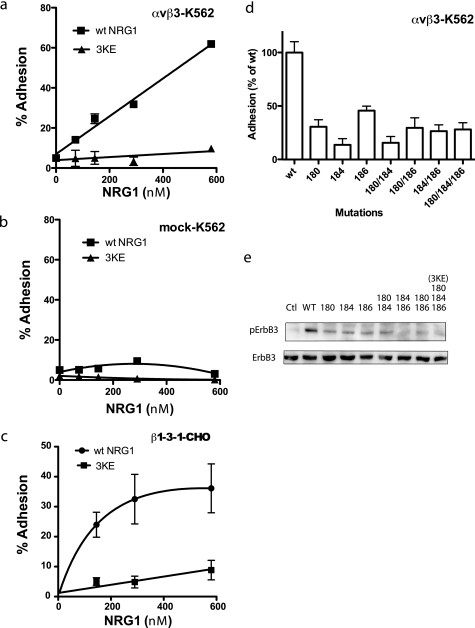
FIGURE 3.
The 3KE mutant is defective in integrin binding. a and b, the 3KE mutant is defective in binding to αvβ3-K562 cells. Adhesion assays was performed using αvβ3-K562 (a) or mock-transfected cells (b) as described in Fig. 1, except that 1 mm MnCl2 was used. The data are shown as means ± S.E. of triplicate experiments. We obtained similar results using β3-CHO cells. c, the 3KE mutant is defective in binding to β1-3-1 integrin. Adhesion assays was performed using β1-3-1-CHO cells as described above. The data are shown as the means ± S.E. of triplicate experiments. The results suggest that the 3KE mutant is defective in binding to αvβ1-3-1, which mimics αvβ3 in ligand binding. d, mutating the Lys residues alone or in combination suppresses integrin binding. Adhesion assays were performed as described above. We coated wells with GST fusion proteins of NRG1 EGF-like domain (580 nm) and used αvβ3-K562 cells. The numbers represent the positions of Lys residues mutated. e, shown is the signaling function of single and double mutants. We determined the ability of the mutants to induce ErbB3 phosphorylation. MCF-7 cells were serum-starved overnight and stimulated with NRG1(10 nm) for 30 min. Cell lysates were analyzed by Western blotting with antibodies specific to phospho-ErbB3 or ErbB3. Ctl, control.