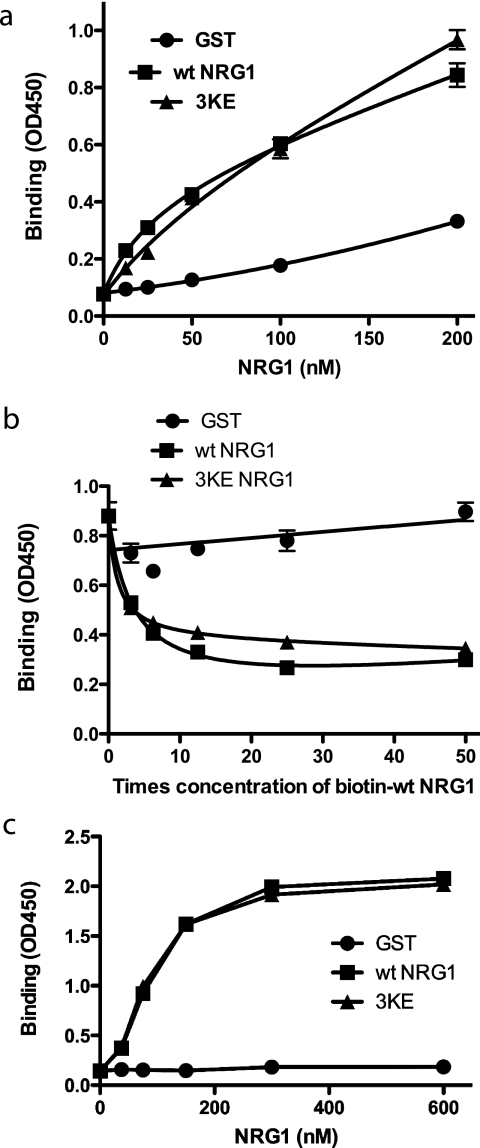
FIGURE 4.
The 3KE mutant of NRG1 binds to ErbB3. a, binding of the 3KE mutant of NRG1 to recombinant ErbB3 is shown. Recombinant soluble ErbB3 Fc fusion protein (R&D system) was coated onto wells of a 96-well microtiter plate (1 μg/ml). NRG1 WT or 3KE mutant was added to the wells and incubated for 1 h at room temperature. GST was used as a control. After washing the wells, bound GST NRG1 was determined by using anti-GST antibody HRP conjugate. The results suggest that the 3KE mutant of NRG1 binds to ErbB3 at levels nearly comparable with WT NRG1. The data are shown as the means ± S.E. of triplicate experiments. b, shown is a competitive binding assay. Recombinant soluble ErbB3 Fc fusion protein was coated onto wells of 96-well microtiter plate (1 μg/ml). Binding of biotin-labeled NRG1 WT (20 nm) in the presence of increasing concentrations of NRG1 WT, 3KE, or GST is shown. After washing the wells, bound biotin-labeled NRG1 WT was determined by using streptavidin HRP conjugate. The data are shown as the means ± S.E. of triplicate experiments. The results suggest that the 3KE mutant of NRG1 binds to ErbB3 at levels comparable with WT NRG1. c, binding of soluble ErbB3-Fc to immobilized NRG1 is shown. We immobilized NRG1 WT and 3KE proteins to the wells of the 96-well microtiter plate at the indicated coating concentrations and incubated with soluble ErbB3-Fc (1 μg/ml) as described above. Bound ErbB3 was detected using HRP-conjugated anti-His6 tag antibodies and peroxidase substrate. ErbB3-Fc has a His6 tag.