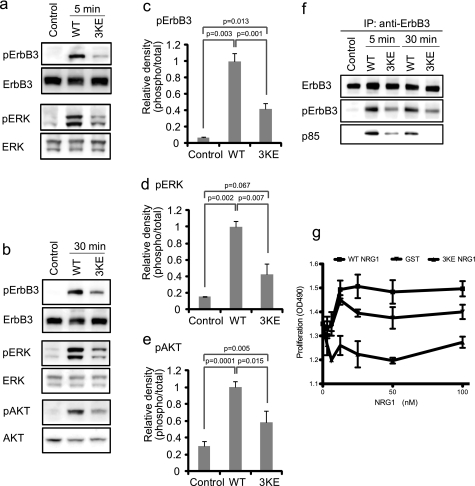
FIGURE 5.
Effect of the 3KE mutation on NRG1 signaling. a and b, the 3KE mutant of NRG1 is defective in inducing ErbB3 phosphorylation, AKT activation, and ERK1/2 activation. MCF-7 cells were serum-starved overnight and stimulated with 10 nm WT and the 3KE mutant of NRG1 for 5 min (a) or 30 min (b). Cell lysates were analyzed by Western blotting. Data are representative of three independent experiments. c–e, levels of phosphorylation were quantified using a luminescence analyzer from triplicate experiments. Data were normalized using WT NRG1 as 1. f, recruitment of p85 of PI3K is shown. Cells were stimulated with WT NRG1 or 3KE, ErbB3 was immuno-purified (IP) from lysates using anti-ErbB3, and immuno-purified materials were analyzed by Western blotting. The p85 subunit of PI3K was detected in lysates of cells stimulated with WT NRG1. Much lower levels of p85 were detected in cells stimulated with 3KE. Data shown are representative of three independent experiments. g, shown is the effect of WT and 3KE NRG1 on the proliferation of MCF-7 cells. Human MCF-7 breast cancer cells were serum-starved overnight and cultured for 48 h with WT or 3KE mutant NRG1. GST was used as a control. Cell number was measured by MTS assays (OD490). The data are shown as the means ± S.E. (n = 3). p < 0.05 by 2-way ANOVA in each case. Data are representative of three independent experiments performed.