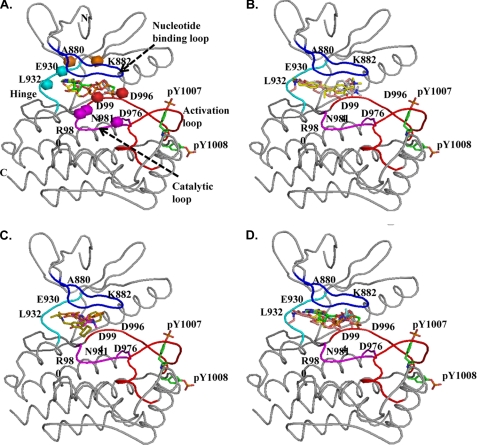
FIGURE 7.
Molecular docking of G6 and its derivatives into the ATP-binding pocket of Jak2. ATP, the ATP analog ACP, G6, and each of its structurally related derivatives were docked into the ATP-binding pocket of the known crystal structure of Jak2 kinase domain (Protein Data Bank code 3E64). A, coiled representation of the structure of Jak2 with ATP and ACP docked into the ATP-binding pocket. The nucleotide binding loop is blue, the hinge region is cyan, the catalytic loop is magenta, and the activation loop is red. Residues within this pocket that are critical for interactions with ATP and other docked drugs have been represented as spheres. Phosphorylation of Tyr1007 within the activation loop is necessary for the activation of kinase activity of Jak2. ATP (yellow) and ACP (green) had strong interactions with the pocket as indicated by their highly negative GRID scores. B, G6 (pink), D28 (gray), and D30 (yellow) docked at the ATP-binding pocket of Jak2. C, D21 (orange), D23 (magenta), and D25 (green) docked at the ATP-binding pocket of Jak2. D, comparison of the docking of ATP (yellow), ATP analog ACP (green), and G6 (pink) with the crystal structure of a Jak2 inhibitor (5B3) (blue) in complex with Jak2 kinase domain showed good correlation (root mean square deviation of <2 Å) between the structures.