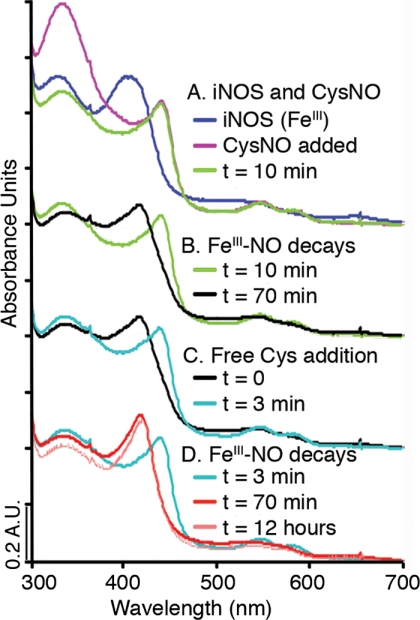
FIGURE 1.
iNOS reactions with NO donor and free thiol, shown by UV-visible spectroscopy. A, reaction of iNOSox (resting state high-spin FeIII, 397 nm, blue) with the NO donor CysNO (purple) forms an FeIII-NO iNOSox derivative (Soret 443 nm, α/β bands 585 nm, 549 nm, green). FeIII-NO is readily distinguishable from FeII-NO spectroscopically by the visible region α/β band absorbances. B, iNOSox FeIII-NO (green) decays to form a low spin Fe species (420 nm, black). C, CysH added to the low spin Fe species (black) re-generates iNOSox FeIII-NO (Soret 443 nm, α/β bands 585 nm, 549 nm, aqua). D, iNOSox FeIII-NO formed by addition of CysH (aqua) decays back to a low spin Fe species (420 nm, red), which is stable for over 12 h (420 nm, pale red).