FIGURE 1.
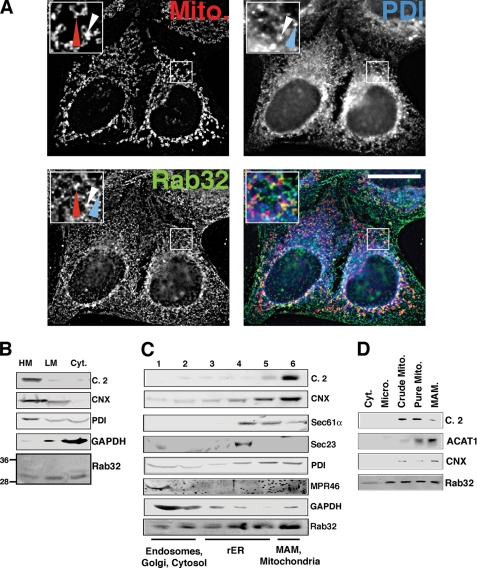
Intracellular localization of Rab32. A, a portion of Rab32 colocalizes with the ER. HeLa cells were grown on coverslips for 24 h and processed for immunofluorescence microscopy. Rab32 was detected with our rabbit polyclonal antibody and PDI with a mouse monoclonal antiserum (ABR, Golden, CO), and mitochondria (Mito) were preloaded with MitoTracker. Insets show a magnified area, indicated by white frames on the bigger pictures. The red arrowheads point out Rab32/mitochondria overlap, and the blue arrowheads point out Rab32/PDI overlap. An example of triple overlap is highlighted by white arrowheads. Scale bar, 25 μm. B, Rab32 fractionation into heavy (HM) and light membranes (LM) and the cytosol (Cyt). Membranes from HeLa cells were fractionated into low and high speed pellets, which were analyzed by Western blot for complex II (mitochondria), calnexin (ER/MAM), PDI (all ER), GAPDH (cytosol), and Rab32 (with molecular masses in kDa are indicated on the left). C, Rab32 distribution upon ER domain fractionation. HeLa cell homogenates were fractionated on a discontinuous 10–30% OptiPrepTM gradient. Marker proteins indicate mitochondria (complex II), MAM (calnexin), rER (Sec61α), transitional ER (Sec23), pan-ER (PDI), endosomes (MPR46), and cytosol (GAPDH). Fractions are assigned their predominant content. D, Rab32 distribution between mitochondria (Mito) and the MAM. HeLa cell homogenates were fractionated according to “Experimental Procedures.” Marker proteins indicate mitochondria (complex 2, C.2) and the MAM (acyl-CoA:cholesterol acyltransferase 1, ACAT1, and calnexin, CNX). Micro, microsomes.