FIGURE 5.
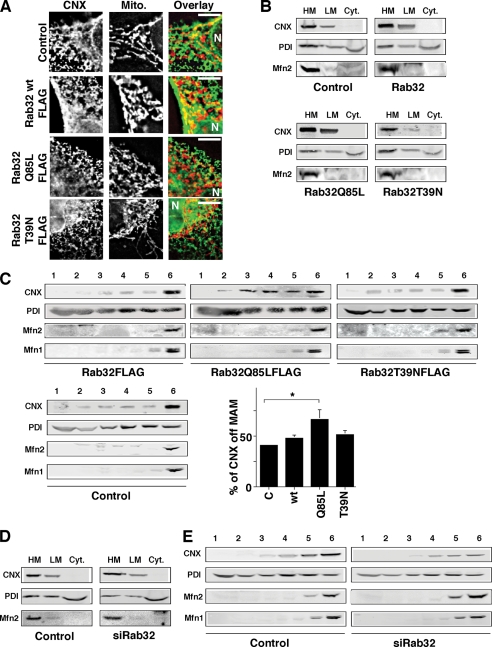
Active Rab32 disrupts the retention of calnexin on the MAM. A, overlap of calnexin (CNX) with mitochondria (Mito) depends on Rab32 activity. HeLa cells were grown on coverslips and transfected with an empty plasmid (pcDNA3) and pcDNA3 containing the cDNA of Rab32FLAG wild type, Rab32FLAGQ85L, Rab32FLAGT39N, and Rab32FLAGL188P. After 48 h, cells were processed for immunofluorescence microscopy, and expressing cells were identified using the FLAG signal (data not shown). Calnexin was detected with our rabbit polyclonal antibody, and mitochondria were preloaded with MitoTracker. Images show portions of cells. The position of the nucleus is indicated by the letter N. Scale bar, 10 μm. B, enrichment of calnexin on heavy membranes (HM) is disrupted by active Rab32Q85L. Membranes from HeLa control cells or cells overexpressing Rab32 and Rab32 mutants as indicated were fractionated into low and high speed pellets and the cytosol, which were analyzed by Western blot for calnexin. LM, light membranes; Cyt, cytosol. C, active Rab32Q85L disrupts calnexin MAM retention. Homogenates from HeLa cells transfected with Rab32FLAG wild type, Q85L, and T39N were fractionated on a discontinuous 10–30% OptiPrepTM gradient. The presence of calnexin was detected by Western blot. Results from three independent fractionations were quantified, and the amounts of calnexin not found in the MAM fractions 5 and 6 were graphed. p = 0.05 between control and Rab32Q85L. D, enrichment of calnexin on heavy membranes is not affected by Rab32 knockdown. Membranes were fractionated into low and high speed pellets and the cytosol and probed for calnexin as in B. E, Rab32 knockdown does not alter the distribution of calnexin on an OptiPrepTM gradient. Homogenates from HeLa cells transfected with scrambled siRNA or Rab32 siRNA were fractionated on a discontinuous 10–30% OptiPrepTM gradient. The presence of calnexin was detected by Western blot.