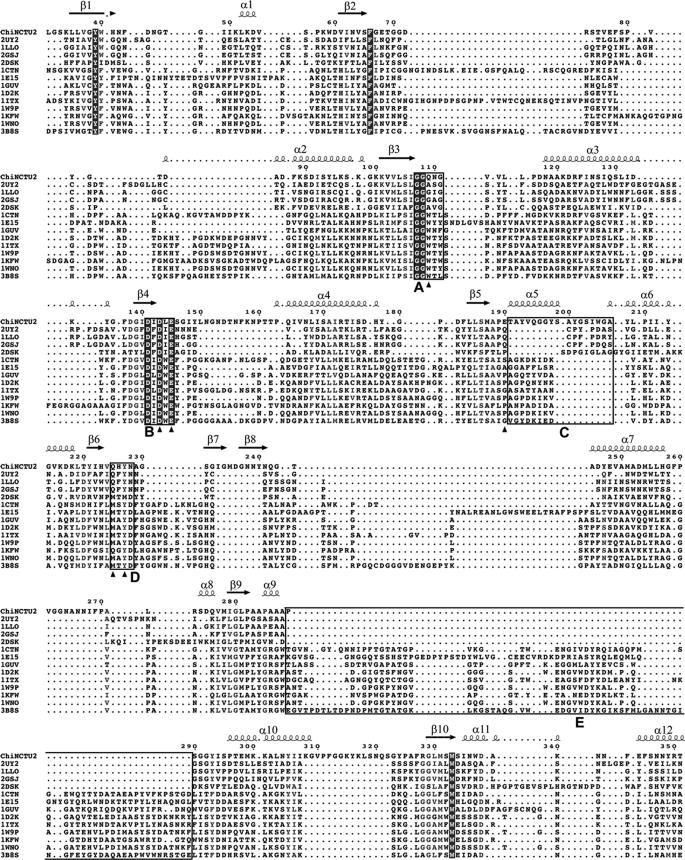
FIGURE 2.
Structural-based multiple-sequence alignment of selected GH-18 chitinases. The sequences of various chitinases with the corresponding PDB codes are aligned with ChiNCTU2 on the similarities of their amino acid sequences (CAZy, available on-line). The endochitinases include Saccharomyces cerevisiae chitinase1 (PDB: 2UY2) (35), H. brasiliensis hevamine (1LLO) (13), Parkia platycephala endochitinase (2GSJ) (36), Pyrococcus furiosus chitinase (2DSK) (37); exochitinases include S. marcescens chitinase A (1CTN) (10), S. marcescens chitinase B (1E15) (38), Homo sapiens chitinase (1GUV) (39), Coccidioides immitis chitinase 1 (1D2K) (40), Bacillus circulans WL-12 chitinase A1 (1ITX) (41), A. fumigatus chitinase B1 (1W9P) (8), Arthrobacter sp. chitinase B (1KFW) (42), A. fumigatus YJ-407 chitinase (1WNO) (with both endo- and exo-activities) (43), and Vibrio harveyi chitinase A (3B8S) (44). The complete conserved residues are blocked in gray. The residues of ChiNCTU2 that were selected for mutagenesis in this work were indicated with arrows. Three boxes (A, B, and D) indicate the highly conserved regions. The box C shows the ChiNCTU2 contains a longer amino acid sequence at this region that forms a helix of α5 (191–199), which exists only in exochitinases, and a loop (200–207). The box E indicates the location of CID.