FIGURE 3.
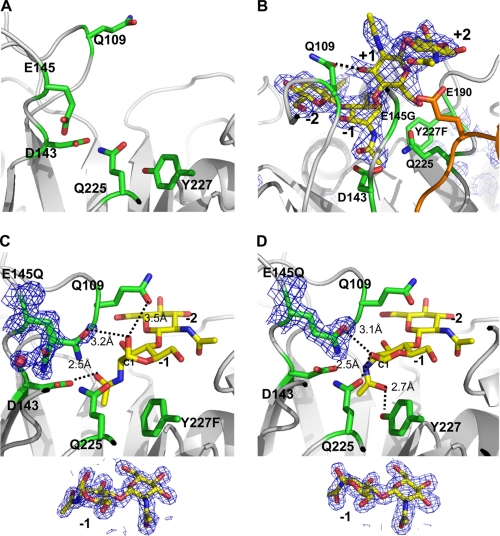
The active site of ChiNCTU2. A, the active site of the native structure is formed with the key residues shown in stick (C, green; N, blue; O, red). The side chain of Glu-145 exhibits the native conformation before substrate binding. B, the complex structure of mutant E145G/Y227F with (NAG)4 reveals four subsites (−2, −1, +1, and +2) (yellow) in the active site. The configuration of (NAG)4 at the −1 subsite is a boat form. The loop between β5∼α5 is shown in orange. C, the complex structure of E145Q/Y227F and (NAG)2 shows that there are −1 and −2-(NAG) in the active site, of which −1-(NAG) exhibits a boat-form conformation that fits well in the electron density below. The N-acetyl group of −1-(NAG) is directed toward Asp-143. The side chain of E145Q with a double conformation might be observed with one interacting with −1-(NAG) and the other coordinating a water molecule. D, the complex structure of E145Q and (NAG)2 shows that −1-(NAG) exhibits a chair-form formation as fits well in the electron density below and directs the N-acetyl group toward Tyr-227 without the Y227F mutation. The electron density 2Fo − Fc map of the substrates and residues E145Q are shown in blue mesh (1 σ) in all figures.