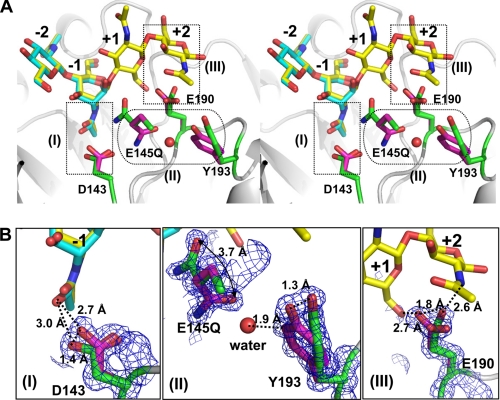
FIGURE 5.
Residues with multiple conformations involved in catalysis in the active site. A, the first conformation (magenta) and the second conformation (green) of residues Asp-143, E145Q, Glu-190, and Tyr-193 are observed in the structure of the mutant E145Q/Y227F complex with (NAG)2 (cyan), shown in stereo view. The (NAG)4 (yellow) from the structure of E145G/Y227F+(NAG)4 complex is superimposed with (NAG)2. B, close views show that the respective residues exhibit double conformations revealed by the electron density 2Fo − Fc map (blue mesh, 1 σ): (I), Asp-143; (II), E145Q and Tyr-193; (III), Glu-190. The color assignment is the same as in A. The interactions between side chains and substrates (or water) are shown in dashed lines with distances.