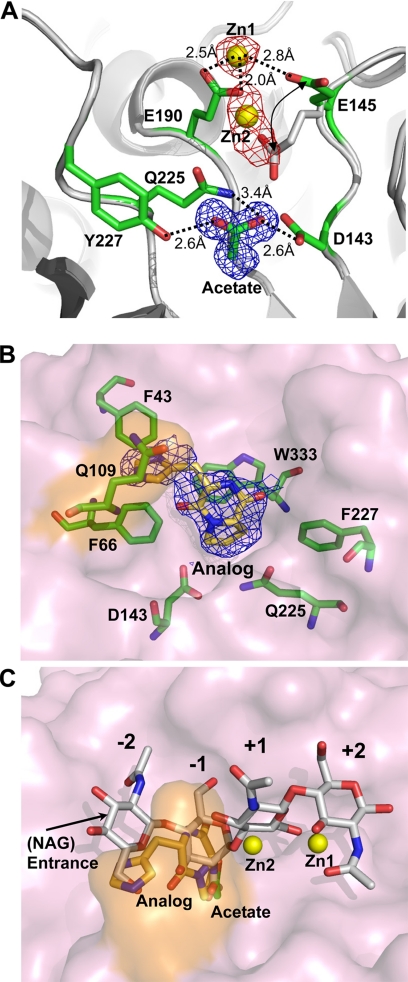
FIGURE 6.
The inhibitors in the active site. A, two zinc atoms (yellow spheres), designed as Zn1 and Zn2 in the structure, are located in the active site and interact with residues Glu-145 and Glu-190. The distance between Zn1 and Zn2 is ∼4 Å. An acetate molecule interacts with Asp-143 and Tyr-227. The interactions are shown in dashed lines labeled with related distances. The 2Fo − Fc density map of the acetate (blue mesh, 1.2 σ) and anomalous-difference Fourier map of zinc atoms (red mesh, 4σ) are shown respectively. The Glu-145 was orientated from un-bond position (white stick) to the zinc-bond position (green sticks). B, the inhibitor cyclo-(l-His-l-Pro) (lemon yellow) bound with the surrounding residues (green sticks) in the active is site shown in a surface plot (light pink), with the important residue Gln-109 on one dynamic loop labeled in orange. The electron density with the 2Fo − Fc coefficient is shown (blue mesh, 0.9 σ). C, the zinc atoms and the inhibitor are superimposed with (NAG)4 from the complex structure to show their related positions.