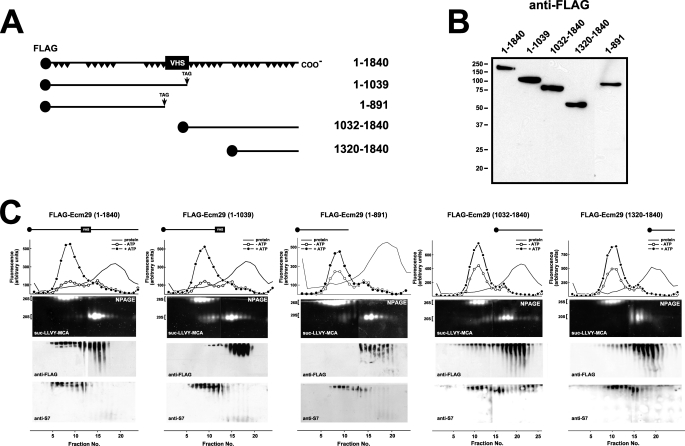
FIGURE 2.
Truncated FLAG-Ecm29 constructs are not associated with the 26 S proteasome after glycerol gradient sedimentation. A, schematic representation of FLAG-tagged Ecm29 constructs. A black circle at the N terminus of each construct represents the FLAG epitope. Black triangles in full-length Ecm29 represent putative HEAT repeats, and the boxed VHS represents a putative VHS domain near the middle of the Ecm29 protein. B, expression of FLAG-tagged Ecm29 constructs in HEK293 cells. Transfected cells were lysed in buffer containing Triton X-100, and 40-μg samples were separated on 10% SDS-PAGE gels followed by immunoblotting with anti-FLAG monoclonal antibodies. The lane containing FLAG-Ecm29(1–891) was inserted from a longer exposure of the membrane as HEK293 cells consistently expressed 5- to 10-fold lower levels of this protein relative to the other constructs. C, glycerol gradient sedimentation of full-length and truncated versions of Ecm29. Post-mitochondrial supernatants (3–4 mg of total protein) from transfected HEK cells lysed in buffer containing sucrose were centrifuged on 4.5-ml 10–30% glycerol gradients and assayed for peptidase activity in the absence (○) or presence (●) of ATP using suc-LLVY-MCA as substrate (top panels). Samples were separated on duplicate native gels (NPAGE), overlaid with suc-LLVY-MCA (second panels), and immunoblotted with anti-FLAG (third panels) or an antibody to 19 S RC subunit S7 (bottom panels). Only one set of fluorescent substrate overlays for each gradient is shown. The immunoblots are composites, because two gels were required to analyze all the fractions from each gradient. Glycerol gradient/native gel analyses demonstrate that FLAG-Ecm29 truncations are not associated with the 26 S proteasome. However, some intact FLAG-Ecm29 molecules, present in fractions 12–18, were free of the 26 S proteasome presumably due to saturation of Ecm29 binding sites by excess recombinant molecules.