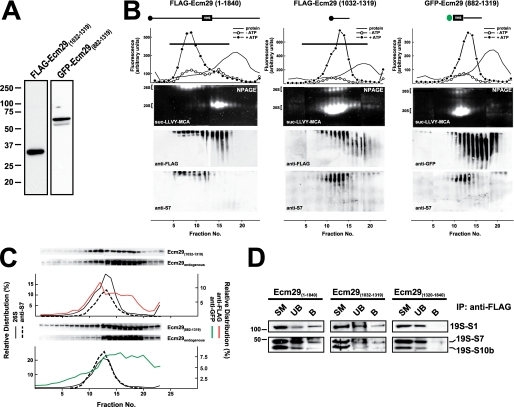
FIGURE 4.
Expression of FLAG-Ecm29(1032–1319) and GFP-Ecm29(882–1319) displaces endogenous Ecm29 and destabilizes the 26 S proteasome. A, expression of FLAG-Ecm29(1032–1319) and GFP-Ecm29(882–1319) in HEK293 cells. SDS-PAGE of Triton X-100 extracts from transfected HEK293 cells (30 μg of protein) were analyzed by immunoblotting with monoclonal antibodies to the FLAG epitope or green fluorescent protein (GFP). B, glycerol gradient sedimentation of FLAG-Ecm29(1032–1319) and GFP-Ecm29(882–1319). The post-mitochondrial supernatants (2–3 mg of protein) from transfected 293 cells lysed in buffer containing sucrose were centrifuged on 4.5-ml 10–30% glycerol gradients and analyzed as shown in Fig. 2C except that one set of the duplicate native gels of the GFP-Ecm29(882–1319) gradient was immunoblotted with a monoclonal anti-GFP antibody (right). For comparison, the glycerol gradient for full-length Ecm29 (FLAG-Ecm29(1–1840)) shown in Fig. 2C has been placed next to the gradients of the two truncated versions of Ecm29. Only one set of fluorescent substrate overlays for each gradient is shown. The immunoblots are composites, because two gels were required to analyze all the fractions from each gradient. Note that the 26 S proteasome is labile and sediments much slower in FLAG-Ecm29(1032–1319) and GFP-Ecm29(882–1319) extracts. C, fractions from the FLAG-Ecm29(1032–1319) and GFP-Ecm29(882–1319) glycerol gradients were separated by SDS-PAGE and immunoblotted with either anti-FLAG (top), or anti-GFP (bottom), or anti-ECM3 (to detect full-length endogenous Ecm29). The relative distribution of the FLAG (red trace) or GFP (green trace) signals on the gradients was estimated by densitometry of the Western blots using the NIH image 1.63f software package, and is expressed as the percentage of the total amount of densitometric units. The relative distribution of the 26 S proteasome (% ATP-dependent activity, solid lines) and S7 (dashed lines) are shown for comparison. The black horizontal lines on the top panel indicate fractions that were pooled and further subjected to immunoprecipitation with M2TM agarose beads. D, full-length FLAG-Ecm29 and FLAG-Ecm29(1032–1319) immunoprecipitate subunits of the 19 S regulatory complex. The anti-FLAG immunoprecipitates of pooled fractions (panel B and data not shown) from the glycerol gradients of full-length FLAG-Ecm29 (fractions 1–14), FLAG-Ecm29(1320–1840) (fractions 1–14), and FLAG-Ecm29(1032–1319) (fractions 1–16) were analyzed by SDS-PAGE and immunoblotting with antibodies to the S1, S7, and S10b subunits of the 19 S RC. The figure shows that only full-length Ecm29 and Ecm29(1032–1319) associate with and precipitate the 19 S RC of the 26 S proteasome.