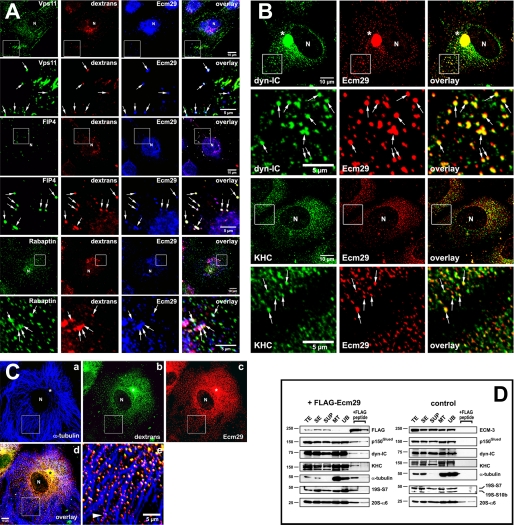
FIGURE 5.
Presence of Ecm29 on endosomes decorated with its putative binding partners. A, Ecm29 is present on vesicles associated with the endocytic proteins Vps11, Rab11-FIP4, and rabaptin. HeLa cells growing on coverslips were exposed to 1 mg/ml Alexa® 568-dextran conjugate (dextrans) for 1 h at 37 °C and then incubated for 5 min in PBS/0.05% saponin prior to fixation with 3% paraformaldehyde. Cells were stained with rabbit anti-ECM2 (Ecm29) and either a mouse monoclonal antibody to Vps11, or a sheep polyclonal antibody to Rab11-FIP4 (FIP4), or a monoclonal antibody to rabaptin. White boxes enclose areas magnified in the panels directly below the full size images. The arrows indicate dextran-filled endosomes on which Ecm29 co-localizes with Vps11, Rab11-FIP4, or rabaptin. B, Ecm29-positive endosomes are bound to kinesin and cytoplasmic dynein. HeLa cells growing on coverslips were fixed with 0.5% glutaraldehyde at 37 °C and stained with ECM2 antibodies (Ecm29) and either a monoclonal antibody to the 70-kDa subunit of cytoplasmic dynein (dyn-IC) or an antibody to kinesin heavy chain (KHC). White boxes enclose areas magnified in the panels directly below the full size images. The arrows indicate examples of Ecm29-positive vesicles bound to the molecular motors. C, Ecm29 is present on endocytic vesicles bound to the microtubule cytoskeleton. HeLa cells growing on coverslips were labeled for 60 min at 37 °C with 5 mg/ml Alexa® 488-dextran conjugate (panel b) and fixed with 0.5% glutaraldehyde. Cells were stained with a mouse monoclonal antibody to α-tubulin (panel a) and anti-ECM2 (Ecm29, panel c) polyclonal antibodies. The merged image from panels a–c is shown in panel d. The arrowhead in panel e indicates a clear example of Ecm29-positive endosomes associated with a microtubule bundle. Samples in panels A–C were visualized with Alexa®-labeled secondary antibodies and confocal microscopy. Asterisk, centrosomal region. N, cell nucleus. Scale bars, 5 or 10 μm as indicated. D, immunoaffinity isolation of microtubule- and molecular motor-bound endosomes. HEK293 cells expressing FLAG-Ecm29 were lysed in buffer containing sucrose and a microtubule (MT)-enriched fraction was prepared in the presence of GTP and Taxol. The MT fraction was treated with nocodazole to disassemble the MTs prior to incubation with M2TM anti-FLAG-agarose beads overnight at 4 °C. The beads were washed, and bound proteins were released with 3× FLAG peptide. Samples of each fraction (20 μg of total protein, except for the samples released from the beads with FLAG peptide, which represent 60 μl or 20% of the total fraction) were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies (left). An MT-enriched fraction was prepared from non-transfected HEK cells as a control and treated as described above (right), except that Ecm29 in each sample was detected with polyclonal anti-ECM3 antibodies. The amount of FLAG-peptide-released protein was estimated by densitometry of the Western blots using the NIH image 1.63f software package and is expressed as the percentage of the amount of each protein found in the cell homogenate: FLAG-Ecm29, 8.7%; p150Glued, 0.5%; dynein intermediate chain (dyn-IC), 0.2%; kinesin heavy chain (KHC), 0.3%; α-tubulin, 1.2%; 19 S RC subunit 7, 1.8%. TE, cell homogenate; SE, soluble extract; SUP, supernatant fraction following centrifugation through a 5% sucrose cushion; MT, microtubule-enriched fraction; and UB, unbound fraction.