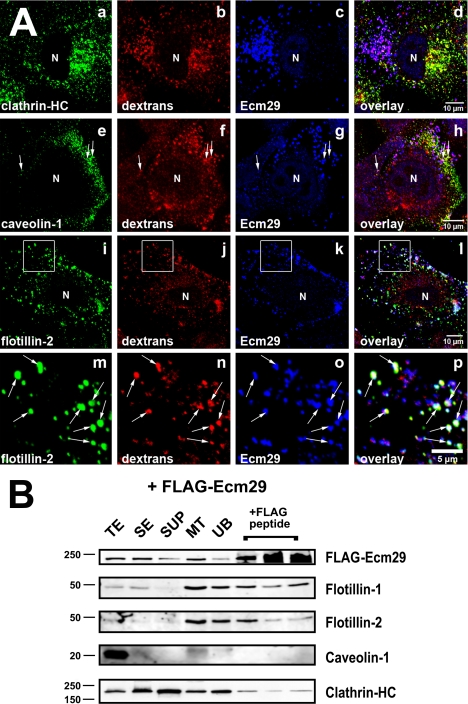
FIGURE 6.
Ecm proteasomes localize to flotillin 2-positive endosomes. A, HeLa cells growing on coverslips were labeled with 1 mg/ml Alexa® 568-dextran conjugate (dextrans) for 1 h at 37 °C and fixed with 2% paraformaldehyde. Cells were stained with rabbit anti-ECM2 (Ecm29), and either monoclonal antibodies to clathrin heavy chain (clathrin-HC), or caveolin-1 (panel e), or a goat polyclonal antibody to flotillin 2 (panels i and m). Samples were visualized with Alexa®-labeled secondary antibodies and confocal microscopy. White boxes (panels i–l) enclose areas magnified in the images directly below each full-size image (panels m–p). Arrows identify examples of dextran-filled, flotillin-2-positive endosomes to which Ecm29 localizes (panels m–p). N, cell nucleus. Scale bars, 5 or 10 μm as indicated. B, Ecm29-positive endosomes bound to microtubules are enriched in flotillins. An MT fraction was prepared as described in the legend to Fig. 5 (see also “Experimental Procedures”); upon disassembly with nocodazole, the fraction was subjected to immunoprecipitation with M2TM anti-FLAG-agarose beads, and bound proteins were released with 3× FLAG peptide. Samples (20 μg of total protein, except for the samples released from the beads, which represent 60 μl or 20% of the total fraction) were analyzed by immunoblotting with the indicated antibodies. The amount of FLAG-peptide-released protein was estimated by densitometry of the Western blots using the NIH image 1.63f software package and is expressed as the percentage of the amount of each protein found in the cell homogenate: FLAG-Ecm29, 2.5%; flotillin-1, 4.3%; flotillin-2, 1.2%; clathrin heavy chain, 0.8%. TE, cell homogenate; SE, soluble extract; SUP, supernatant fraction following centrifugation through a 5% sucrose cushion; MT, microtubule-enriched fraction; and UB, unbound fraction.